Abstract
Purpose
Complete mesocolic excision improves lymphadenectomy for right hemicolectomy and respects the embryological planes. However, its effect on cancer-free and overall survival is questioned. Therefore, we aimed to determine the potential benefits of the technique by performing a systematic review of the literature and meta-analysis of the available evidence.
Methods
Web of Science, PubMed/Medline, and Embase were searched on February 22, 2023. Original studies on short- and long-term oncological outcomes of adult patients undergoing right hemicolectomy with complete mesocolic excision as a treatment for primary colon cancer were considered for inclusion. Outcomes were extracted and pooled using a model with random effects.
Results
A total of 586 publications were identified through database searching, and 18 from citation searching. Exclusion of 552 articles left 24 articles for inclusion. Meta-analysis showed that complete mesocolic excision increased the lymph node harvest (5 studies, 1479 patients, MD 9.62, 95% CI 5.83–13.41, p > 0.0001, I2 84%), 5-year overall survival (5 studies, 2381 patients, OR 1.88, 95% CI 1.14–3.09, p = 0.01, I2 66%), 5-year disease-free survival (4 studies, 1376 patients, OR 2.21, 95% CI 1.51–3.23, p < 0.0001, I2 0%) and decreased the incidence of local recurrence (4 studies, 818 patients, OR 0.27, 95% CI 0.09–0.79, p = 0.02, I2 0%) when compared to standard right hemicolectomy. Perioperative morbidity was similar between the techniques (8 studies, 3899 patients, OR 1.04, 95% CI 0.89–1.22, p = 0.97, I2 0%).
Conclusion
Meta-analysis of observational and randomised studies showed that right hemicolectomy with complete mesocolic excision for primary right colon cancer improves oncologic results without increasing morbidity/mortality. These results need to be confirmed by high-quality evidence and randomised trials in selected patients to assess who may benefit from the procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1986, Heald and colleagues from Basingstoke revolutionised the outcomes of rectal cancer surgery by reporting total mesorectal excision (TME) [1]. This standardized technique combines proctectomy with complete removal of the mesorectum following the embryological planes, therefore allowing a complete lymphadenectomy of the first lymph node stations, and led to major improvements in overall survival (OS) and local recurrence [2].
In 2009, Hohenberger proposed applying a similar approach to right-sided colon cancer and introduced the concept of complete mesocolic excision (CME). The aim was to improve oncological outcomes by following the hypothesis that an increased number of harvested lymph nodes and complete removal of the mesocolon correlates with improved survival [3].
It is noteworthy that Yamaoka et al. reported that the incidence of lymph node metastases in patients with right-sided colon cancer was 41.5%. These lymph node metastases were present in the D2 lymph nodes in 9.7% of patients and in D3 lymph nodes in 1.5% of patients [4]. However, D3 lymph nodes located along the superior mesenteric vein are not removed when performing conventional (D2) right hemicolectomy. Therefore, to improve the lymph node yield and remove these nodes, CME is based on three concepts: 1. Sharp embryological plane dissection to completely remove the mesocolon, including all the lymph nodes draining the tumour, 2. Central vascular ligation to remove central lymph nodes and 3. Sufficient bowel resection to be able to remove the pericolic lymph nodes and the adjacent vascular arcades [5, 6]. This partially overlaps with the recommendation from Asian professional societies, notably the Japanese Society for Cancer of the Colon and Rectum, which recommends D3 lymphadenectomy (LND3) for ≥ cT2 colorectal cancer [7].
A recent consensus statement article by Tejedor et al. showed that the main characteristics of the procedure to qualify for CME are central vascular ligation, exposure of the superior mesenteric vein and intact mesocolon excision [8].
Complete mesocolic excision could therefore theoretically improve overall and disease-free survival for selected patients with right-sided colon cancer. However, implementation of this procedure has not been universally adopted, because doubts have been raised concerning a potential increase in the perioperative morbidity of the procedure, notably central vascular injuries, and also as a result of its increased technical difficulty. Therefore, we aimed to assess the current state of the literature to determine if CME in right hemicolectomy has better oncological outcomes when compared to non-CME procedures and if it has an impact on perioperative morbidity/mortality.
Methods
The present methodology follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [9]. The study protocol was registered in the PROSPERO database (CRD42021225411).
Literature search
Medline, Embase, and Web of Science were searched on 22 February 2023. The literature search strategy is reported in Table 1. Additional records were identified by screening the reference lists of existing reviews in the field.
Study selection: inclusion and exclusion criteria
In this article, we use the term CME to define the technique reported by Hohenberger et al. [6]. Conversely, we use the term non-CME to designate the study control groups defined by the use of standard right hemicolectomy. D2 lymphadenectomy (LND2), the conventional procedure used in most centres in our studies, is defined by the Japanese Society for Cancer of the Colon and Rectum and requires the removal of the pericolic and intermediate lymph nodes [10]. To be included, publications had to be original studies reporting on short- and long-term oncological and non-oncological outcomes of adult patients undergoing right hemicolectomy with CME as a curative treatment for primary colorectal cancer. Studies including fewer than 50 patients, those which included patients with metastatic disease, those without a control group (with non-CME right hemicolectomy), the grey literature, or any kind of secondary analysis (meta-analyses, systematic reviews, or duplicate patients) were excluded. No language restriction was applied.
Data extraction and outcomes
Two reviewers (GDL, JM) independently selected articles for inclusion and extracted the data according to a pre-established data collection form. Any discrepancies were solved by reaching a consensus between the two reviewers. The following data were extracted: first author, publication year, the country where the investigation took place, study period, study design, TNM inclusion criteria, number of patients, number of patients who underwent CME, sex, age, surgical technique, postoperative pain, time to passage of flatus, time to diet, blood loss, number of harvested lymph nodes, length of hospital stay, postoperative complications, conversion rate, reoperation rate, 30-day mortality, 3-year and 5-year OS, 3-year and 5-year disease-free survival (DFS), local recurrence, metastatic recurrence, and overall recurrence.
Risk of bias assessment of included studies
The Cochrane risk of bias tool was used to assess the quality and risk of bias for the included randomised controlled study [11]. The methodological quality of all included non-randomised studies was assessed on the basis of the Newcastle–Ottawa Scale [12]; the studies that scored ≥ 7 were considered to be of high quality. This tool was used to assess each study for eight parameters and categorised into three groups: first, the selection of the study groups; second, the comparability of the groups; and third, the ascertainment of either the exposure or outcome of interest for case–control studies. One point was awarded for each quality item. High-quality studies were awarded up to 9 points [12] (Table 1). Funnel plots were used to check the risk of publication bias (Supplemental material 1).
Statistical analysis
Qualitative data were summarized in tables. For studies that only provided median and interquartile range, conversion into mean was done using the method proposed by Liu et al. [13] and standard deviation with the method from Wan et al. [14]. Quantitative analysis was performed using the ReviewManager 5.4.1 software (The Cochrane Collaboration, The Nordic Cochrane Center, Copenhagen, Denmark). Odds ratios with 95% CI were reported. Model with random effects was applied (DerSimonian and Laird’s approach). Heterogeneity was quantified using the I2 value. The results of meta-analyses are shown as forest plots. Statistical significance was defined as p < 0.05.
Results
Study selection process
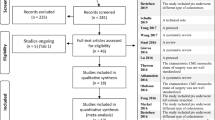
A total of 209 publications were identified on Web of Science, 200 on Pubmed/Medline, and 159 on Embase. Eighteen publications were identified from citation searching. Two hundred and ten duplicates were removed. Of the 376 publications that were identified as eligible, 260 were excluded after title/abstract screening and 92 after the full-text screening, based on our inclusion/exclusion criteria. Ultimately, 24 publications were included in the qualitative and quantitative review (Fig. 1).
Characteristics of included studies
Included studies were conducted in Europe and Asia. These articles were recent; 15 were published in the last 5 years [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29], and none before 2013. Data from three studies were prospectively collected [17, 24, 28], two were retrospective case–control studies [15, 16], 18 were retrospective cohort studies [18, 19, 21,22,23, 25,26,27, 29,30,31,32,33,34,35,36,37,38], and one was a randomised controlled trial [20]. Thirteen compared surgical techniques for CME [25,26,27,28,29, 31,32,33,34,35,36,37,38]. All studies included patients with right colon cancer stages T1 to T4, N0 to N2, and without any known distant metastases. TNM stages ranged between I and III. Three studies included only laparoscopic surgery [15, 19, 20], and one study only open surgery [30]. Newcastle–Ottawa Scale scores ranged between 6/9 and 8/9. Cochrane Risk of Bias assessment for our included randomised controlled trial showed a high risk of bias for blinding of participants and personnel, low risk of bias for random sequence generation, blinding of outcome assessment, selective outcome reporting and other sources of bias, and an unclear risk of bias for allocation concealment and incomplete outcome data. Data from studies comparing CME vs non-CME are summarized in Table 2.
Short-term outcomes
The data on short-term outcomes are summarized in Table 3.
Operative time
Two studies reported no significant difference in terms of operative time [15, 19], two studies reported an increased operative time for the CME group [20, 23], and one reported a decrease in operative time for the CME group [21]. In the quantitative meta-analysis, no difference was found in terms of mean operative time between the CME and the non-CME group (5 studies, 1689 patients, mean difference (MD) − 0.63, 95% confidence interval (CI) − 19.37–18.11, p = 0.95, I2 91%) (Fig. 2a).
Meta-analysis of CME versus non-CME. Forest plots of short-term outcomes comparing CME versus non-CME. Each horizontal bar summarizes a study. The bars represent 95% confidence intervals. The squares inform each study’s weight in the meta-analysis. The diamond in the lower part of the graph depicts the pooled estimate along with 95% confidence intervals. Odds ratio (OR) and mean difference (MD) were obtained using models with random effect (Mantel–Haenszel). Heterogeneity was assessed using the Q-test and quantified using the I2 value
Perioperative blood loss
Two studies reported a statistically significant decrease in blood loss in the CME group [15, 19]. Two other studies reported no significant difference in this outcome [20, 23].
According to our meta-analysis, there was no difference in mean blood loss between the CME and the non-CME group (4 studies, 1397 patients, MD − 25.33, 95% CI − 50.73–0.07, p = 0.05, I2 92%) (Fig. 2b).
Conversion to open surgery
Five studies reported the incidence of conversion to open surgery. The incidence of conversion to open surgery ranged between 1% and 14% for the non-CME group [17, 20], and between 0% and 21% for the CME group [17, 20]. Meta-analysis found that patients who underwent CME were at increased risk of conversion to open surgery (5 studies, 2234 patients, OR 1.57, 95% CI 1.04–2.37, p = 0.03, I2 0%) (Fig. 2c).
Lymph node harvest
All studies comparing CME versus non-CME reported a statistically significant increase in harvested lymph nodes in favour of the CME technique, with mean values ranging between 22.6 and 57.9 lymph nodes for the CME group and between 14.0 and 50.4 lymph nodes for the non-CME group [15, 17, 19,20,21,22,23,24, 30]. Meta-analysis found that CME increased the number of harvested lymph nodes by 9 lymph nodes when compared to non-CME (9 studies, 3955 patients, MD 9.03, 95% CI 5.36–12.71, p < 0.00001, I2 95%) (Fig. 2d).
Incidence of reoperation
Two studies reported the incidence of reoperation, which ranged between 5.3% and 7.5% for the non-CME group, and between 7.2% and 8.9% for the CME group. These differences were not statistically significant [24, 30]. Pooled analysis did not show any difference in terms of the incidence of reoperation between the CME and the non-CME group (2 studies, 1111 patients, OR 1.24, 95% CI 0.81–1.92, p = 0.32, I2 0%) (Fig. 2e).
Time to first passage of flatus
One study reported time to passage of flatus, with a mean value of 3.1 days (non-CME) versus 2.9 days (CME); this difference was not statistically significant [19].
Length of hospital stay
Seven studies reported the length of hospital stay, without showing a statistically significant difference between the techniques: CME groups ranged between 6.4 and 13.1 days, and non-CME groups ranged between 6.4 and 16.7 days [15, 19,20,21,22,23,24]. Quantitative analysis did not show any difference between CME and non-CME in terms of length of hospital stay (7 studies, 2780 patients, MD − 0.83, 95% CI − 0.9–0.17, p = 0.18, I2 54%) (Fig. 2f).
Incidence of postoperative complications
Eight studies reported similar incidences of postoperative complications such as anastomotic leak, chylous ascites, pneumonia, bleeding, small bowel obstruction, wound infection, venous thromboembolism and urinary tract infections between CME (incidence ranging between 12.1% and 31.3%) and non-CME (incidence ranging between 16.7% and 35.9%) [15, 17, 19,20,21,22,23,24]. One study reported a median follow-up for postoperative complications of 30 days [20]. One study followed patients for complications for 60 days [17], and another study for 90 days [21]. The timing for follow-up was not reported in 5 studies [15, 16, 19, 22, 24]. According to the meta-analysis, there was no difference in terms of the incidence of postoperative complications between the CME and the non-CME group (8 studies, 3899 patients, OR 1.04, 95% CI 0.89–1.22, p = 0.97, I2 0%) (Fig. 2g). The funnel plot for this outcome shows a symmetrical distribution (Supplemental material 1A).
Incidence of 30-day mortality
The incidence of 30-day mortality was reported in eight articles ranging between 0% and 5% for both the CME and the non-CME groups [17, 19,20,21, 23, 24, 30], with no significant difference. Quantitative analysis did not show any difference in terms of 30-day mortality rates between the CME and the non-CME group (8 studies, 3869 patients, OR 1.03, 95% CI 0.56–1.92, p = 0.92, I2 0%) (Fig. 2h).
Long-term outcomes
The data on long-term outcomes is summarized in Table 4.
Three-year overall survival
Two studies reported an increase in 3-year OS: Ouyang et al. reported 93.5% (CME) versus 85.0% (non-CME) (p = 0.017) [19], and Khan et al. reported 99% (CME) versus 90% (non-CME) (p = 0.0454) [23]. One other study reported no significant difference [21].
In the quantitative meta-analysis, no difference was found in terms of 3-year OS between the CME and the non-CME group (3 studies, 579 patients, OR 1.79, 95% CI 0.72–4.43, p = 0.21, I2 44%) (Fig. 3i).
Meta-analysis of CME versus non-CME. Forest plots of long-term outcomes comparing CME versus non-CME. Each horizontal bar summarizes a study. The bars represent 95% confidence intervals. The squares inform each study’s weight in the meta-analysis. The diamond in the lower part of the graph depicts the pooled estimate along with 95% confidence intervals. Risk ratio (RR) was obtained using models with random effect (Mantel–Haenszel). Heterogeneity was assessed using the Q-test and quantified using the I2 value
Three-year disease-free survival
Three-year DFS was greater in the CME group in one study, with a reported 91.6% (CME) versus 80.0% (non-CME) (p = 0.014) [19]. Two studies reported no significant difference [21, 23].
Pooled analysis did not show any difference in terms of the incidence of 3-year DFS between the CME and the non-CME group (3 studies, 579 patients, OR 1.81, 95% CI 0.96–3.45, p = 0.7, I2 30%) (Fig. 3j).
Five-year overall survival
Two studies reported an increase in 5-year OS for the CME group: 100% (CME) versus 89.49% (non-CME) (p = 0.049) [15], and 89.4% (CME) versus 71.5% (non-CME) (p = 0.011) [30]. Three studies reported no difference between the two groups for this outcome [17, 22, 24].
Meta-analysis found that patients who underwent CME showed improved 5-year OS (5 studies, 2381 patients, OR 1.87, 95% CI 1.13–3.09, p = 0.02, I2 66%) (Fig. 3k). The funnel plot for this outcome shows an asymmetrical distribution, indicating potential publication bias (Supplemental material 1B).
Five-year disease-free survival
Five-year DFS was similar for both groups in two studies [15, 22], but two studies reported results in favour of the CME group: 93.8% (CME) versus 78% (non-CME) (p = 0.049) [30], and 89.9% (CME) versus 82.0% (non-CME) (p = 0.00028) [17].
Pooled analysis showed an improvement in terms of 5-year DFS for the CME group (4 studies, 1376 patients, OR 1.95, 95% CI 1.35–2.82, p = 0.0004, I2 0%) (Fig. 3l). The funnel plot for this outcome a shows symmetrical distribution (Supplemental material 1C).
Incidence of local recurrence
All four studies reporting incidence of local recurrence showed no significant difference [19, 21,22,23].
Quantitative analysis showed improved incidence in terms of local recurrence for the CME group (4 studies, 666 patients, OR 0.24, 95% CI 0.08–0.71, p = 0.01, I2 0%) (Fig. 3m).
Incidence of distant recurrence
One study reported an improved incidence in distant recurrence, with 8% (CME) versus 24% (non-CME) (p = 0.026) [23], whilst three studies no significant difference for this outcome [19, 21, 22].
Pooled analysis showed no difference in the incidence of distant recurrence between the CME and the non-CME group (4 studies, 666 patients, OR 0.60, 95% CI 0.32–1.10, p = 0.10, I2 23%) (Fig. 3n).
Discussion
Studies comparing CME and non-CME included in this review have tended to show that CME increases the number of lymph nodes harvested, without increasing postoperative complications, or 30-day mortality. The incidence of conversion to open surgery seemed to be increased in the CME group, but this may be due to one included study where authors reported higher overall conversion rates as a result of the implementation of laparoscopic surgery in their centres [17]. The ongoing randomised controlled trial included in our review, named the RELARC trial, has for the moment only published preliminary short-term outcomes but will release long-term outcomes in the future. The authors concluded that CME did not increase perioperative complications of right hemicolectomy compared to non-CME but, when looking at the detailed results, the incidence of vascular injury was significantly increased (3% for CME versus 1% for non-CME, p < 0.05) [20]. A randomised clinical trial in Russia comparing short-term outcomes for CME versus non-CME (COLD) showed no difference in mortality or complication rates [39], and another prospective non-randomised controlled trial in China showed no difference in perioperative morbidity/mortality [40]—these studies did, however, include both left- and right-sided resections. As CME is technically challenging to perform, favourable short-term outcomes depend on the experience and technique of the surgical team, as shown by Bernhoff et al. [16].
Regarding long-term outcomes, our meta-analysis showed improved local recurrence rates and an increase in 5-year OS and 5-year DFS. Bertelsen et al. also reported a risk reduction in 5-year OS, but only in their 2010–2013 subgroup, which suggests the impact of a learning curve for this procedure. They also showed risk reduction in 5-year DFS, especially for the higher stages, and once again more marked in the most recent subgroup [17]. The recent prospective study by Benz et al. which included 1004 patients has shown no significant increase in 5-year OS. However, in patients with stage III disease, the authors did report a significant increase in this outcome (78.3% CME versus 65.0% non-CME). It is also worth mentioning that this study included pathological specimen control by mesenteric area to further determine if the technique was successfully performed [24]. A prospective study by Gao et al. showed improved 3-year local recurrence-free survival (100% CME vs 90.2% non-CME) but did include both left- and right-sided disease [40].
Long-term impact on bowel function was assessed by Bertelsen et al., who did not find an increased risk of bowel dysfunction, long-term pain or impaired quality of life when compared to non-CME [18].
Recent data concerning preoperative staging have shown that computed tomography lacks accuracy, especially in predicting lymph node metastases, with a sensitivity of 57% and a specificity of 66%. This has an impact on disease management in general but also on the potential choice of surgical technique [41].
To our knowledge, CME versus non-CME for right hemicolectomy had previously been analysed in several systematic reviews with meta-analyses, the most recent ones being published in 2021. In their analysis, Balciscueta et al. included 29 studies and obtained a complication rate of 17.4% (CME group) versus 19.4% (non-CME) (p = 0.84), and a 5-year OS of 78.2% (CME) versus 67.1% (non-CME) (p = 0.03). They subclassified OS according to stage II and stage III disease, which showed a greater advantage for the CME group [42]. De Simoni et al. included 8 studies in their meta-analysis and obtained no difference between techniques for complication rates (odds ratio 1.13, p = 0.34). However, they showed a significant benefit in terms of 3-year OS (odds ratio 1.57, p = 0.003) and 5-year OS (odds ratio 1.41, p = 0.02) in favour of CME [43]. Anania et al. included 17 studies in their meta-analysis and obtained no difference between techniques for complication rates (relative risk 0.82, CI 0.67–1.00). However, they showed a significant benefit in terms of 3-year OS (relative risk 0.42, 95% CI 0.27–0.66) and 5-year OS (relative risk 0.36, 95% CI 0.17–0.56) in favour of CME [44]. Díaz-Vico et al. included 27 studies and reported a higher 3-year OS (relative risk 1.09, p = 0.01) and 5-year OS (relative risk 1.05, p = 0.02) for CME, without any increase in the complication rate (relative risk 1.13, p = 0.58) [45]. Ferri et al. included 17 studies and reported a complication rate of 21% (CME group) versus 24% (non-CME) (p = 0.66), 5-year DFS of 81.4% (CME) versus 79.7% (non-CME) (p < 0.01), and a 5-year OS of 86% (CME) versus 70.8% (non-CME) (p < 0.01) [46]. All these meta-analyses of non-randomised studies shared the conclusion that CME improves DFS, OS, and local and metastatic recurrence when compared to non-CME, whilst showing similar postoperative complication rates, which is a trend also shown in our study.
Our systematic review and meta-analysis on short- and long-term outcomes of CME is to our knowledge the first to include only comparative studies with non-metastatic patients specifically undergoing right hemicolectomy. Our review also benefits from a large patient sample, including the most recent studies. Since the last systematic review/meta-analysis, we have identified four new cohort studies and one new randomised controlled trial. The main strength of this review is its thorough evaluation of CME in terms of short- and long-term outcomes.
Conceptually, CME is similar to another procedure used since 1977 in Asia, notably in Japan: right hemicolectomy with LND3. Although conceptually similar, there are a few differences between LND3 and CME. First, CME involves strict embryological plane dissection rather than removing central lymph node stations, which sometimes involves the removal of the lymph nodes around the head of the pancreas or the gastroepiploic arcade. Second, LND3 requires at least 10 cm resection margins, but CME is usually more extensive because of the requirement to remove adjacent vascular arcades. Third, LND3 usually spares the root of the main colic vessels because the ligation only occurs at the root of the primary feeding vessels, whereas CME mandates more radical central vascular ligation and dissection up to the root of the main colic vessels [47].
The main limitations of this review are the following: first, data on right hemicolectomy with CME are still scarce and not of the highest quality; second, almost all included studies are cohort studies, which may carry a risk of selection bias; third, publication bias may be present; and finally, the quality evaluation of the pathological specimens is heterogeneous and not always reported. This makes it difficult to correctly assess if the patient benefited from the maximum effect of CME and, on the other hand, many supposed conventional non-CME procedures may have been more/less extensive than the standard LND2, as has been outlined by Bertelsen et al. reporting suboptimal survival rates in the control group for stage I and II disease [48]. Future randomised controlled trials should include quality control by expert surgeons not only for CME but also for the control group. On this aspect, Siani et al. proposed a classification for pathological evaluation, which could be useful for future research [49, 50]. It is also worth noting that surgeons practising CME are usually more experienced and can therefore report lower incidences of postoperative complications—this raises a word of caution as the widespread implementation of the technique may lead to an increase in postoperative morbidity and severe complications including central vascular injuries. Finally, the use of (neo-)adjuvant therapy and other important variables were not systematically reported, which could introduce a bias in terms of survival outcomes. It is also worth noting that the biology of colorectal cancer is still not fully understood and that survival is not solely based on lymph node involvement—other factors, such as extramural venous invasion may also play a role in the prognosis of the disease [51]. This review also does not take into account the emerging role of immunotherapy in digestive surgery [52].
The fact that improved lymphadenectomy appears to improve survival has classically been attributed to the stage migration phenomenon, but some authors are suggesting this may also be a reflection of molecular features of the cancer [53]. This bias may also have been present in our included studies. Detection bias is limited by homogenous and standardized follow-up, which is reported in most included studies.
As outlined, the ongoing RELARC trial will soon be publishing long-term survival results. These authors are waiting to release long-term results to determine if survival rates could be improved, which would constitute a justification for the increased complexity of the CME technique [20]. A multicentre randomised prospective study comparing CME versus non-CME is currently recruiting in Italy (CoME-IN) and will report short- and long-term outcomes [54], and a prospective multicentre cohort study in South Korea is evaluating the oncologic outcomes of laparoscopic modified CME (PIONEER).
Conclusion
Current pooled evidence of observational and randomised studies suggests that CME for right hemicolectomy improves the lymph node yield, 5-year OS and 5-year DFS when compared to non-CME, with similar perioperative morbidity. Similarly, the incidence of recurrence is decreased. However, a recent randomised controlled trial, pooled in our meta-analysis, questions the incidence of perioperative complications, and showed a significant incidence of central vascular injuries. These results require confirmation by high-quality evidence, such as multicentre randomised controlled trials with long-term follow-up.
Data availability
All data generated or analysed during this study are included in this published article.
References
Heald RJ, Ryall RDH (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 327:1479–1482. https://doi.org/10.1016/S0140-6736(86)91510-2
Heald RJ, Moran BJ, Ryall RDH et al (1998) Rectal cancer: the basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 133:894–898. https://doi.org/10.1001/archsurg.133.8.894
Chen SL, Bilchik AJ (2006) More extensive nodal dissection improves survival for stages I to III of colon cancer. Ann Surg 244:602–610. https://doi.org/10.1097/01.sla.0000237655.11717.50
Yamaoka Y, Kinugasa Y, Shiomi A et al (2017) The distribution of lymph node metastases and their size in colon cancer. Langenbecks Arch Surg 402:1213–1221. https://doi.org/10.1007/s00423-017-1628-z
Benz S, Tannapfel A, Tam Y et al (2019) Proposal of a new classification system for complete mesocolic excison in right-sided colon cancer. Tech Coloproctology 23:251–257. https://doi.org/10.1007/s10151-019-01949-4
Hohenberger W, Weber K, Matzel K et al (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation-technical notes and outcome. Colorectal Dis. 11:354–364. https://doi.org/10.1111/j.1463-1318.2008.01735.x
Colon JS (2019) Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d English edition [secondary publication]. J Anus Rectum Colon. 3:175–195. https://doi.org/10.23922/jarc.2019-018
Tejedor P, Francis N, Jayne D et al (2022) Consensus statements on complete mesocolic excision for right-sided colon cancer technical steps and training implications. Surg Endosc 36:5595–5601. https://doi.org/10.1007/s00464-021-08395-0
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10:89. https://doi.org/10.1186/s13643-021-01626-4
Watanabe T, Muro K, Ajioka Y et al (2018) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23:1–34. https://doi.org/10.1007/s10147-017-1101-6
Higgins JPT, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 343:d5928. https://doi.org/10.1136/bmj.d5928
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. https://doi.org/10.1007/s10654-010-9491-z
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Meth Med Res 27:1785–1805. https://doi.org/10.1177/0962280216669183
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Meth 14:135. https://doi.org/10.1186/1471-2288-14-135
An MS, Baik H, Oh SH et al (2018) Oncological outcomes of complete versus conventional mesocolic excision in laparoscopic right hemicolectomy. ANZ J Surg 88:E698–E702. https://doi.org/10.1111/ans.14493
Bernhoff R, Sjövall A, Buchli C et al (2018) Complete mesocolic excision in right-sided colon cancer does not increase severe short-term postoperative adverse events. Colorectal Dis 20:383–389. https://doi.org/10.1111/codi.13950
Bertelsen CA, Neuenschwander AU, Jansen JE et al (2019) 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol 20:1556–1565. https://doi.org/10.1016/S1470-2045(19)30485-1
Bertelsen CA, Larsen HM, Neuenschwander AU et al (2018) Long-term functional outcome after right-sided complete mesocolic excision compared with conventional colon cancer surgery: a population-based questionnaire study. Dis Colon Rectum 61:1063–1072. https://doi.org/10.1097/DCR.0000000000001154
Ouyang M, Luo Z, Wu J et al (2019) Comparison of outcomes of complete mesocolic excision with conventional radical resection performed by laparoscopic approach for right colon cancer. Cancer Manag Res 11:8647–8656. https://doi.org/10.2147/CMAR.S203150
Xu L, Su X, He Z et al (2021) Short-term outcomes of complete mesocolic excision versus D2 dissection in patients undergoing laparoscopic colectomy for right colon cancer (RELARC): a randomised, controlled, phase 3, superiority trial. Lancet Oncol 22:391–401. https://doi.org/10.1016/S1470-2045(20)30685-9
Giani A, Bertoglio C, Mazzola M et al (2022) Mid-term oncological outcomes after complete versus conventional mesocolic excision for right-sided colon cancer: a propensity score matching analysis. Surg Endosc Interv Tech 36:6489–6496. https://doi.org/10.1007/s00464-021-09001-z
Tümay LV, Güner OS, Batı İB, Zorluoğlu A (2020) Is complete mesocolic excision technique superior to conventional hemicolectomy technique for patients with right-sided colon cancer? Preliminary findings from a single-center retrospective analysis. Turk J Colorectal Dis 30:301–310. https://doi.org/10.4274/tjcd.galenos.2020.2020-8-4
Khan JS, Ahmad A, Odermatt M et al (2021) Robotic complete mesocolic excision with central vascular ligation for right colonic tumours - a propensity score-matching study comparing with standard laparoscopy. BJS Open. https://doi.org/10.1093/bjsopen/zrab016
Benz SR, Feder IS, Vollmer S et al (2022) Complete mesocolic excision for right colonic cancer: prospective multicentre study. Br J Surg. https://doi.org/10.1093/bjs/znac379
He Z, Zhang S, Xue P et al (2019) Completely medial access by page-turning approach for laparoscopic right hemi-colectomy: 6-year-experience in single center. Surg Endosc 33:959–965. https://doi.org/10.1007/s00464-018-6525-1
Ceccarelli G, Costa G, Ferraro V et al (2021) Robotic or three-dimensional (3D) laparoscopy for right colectomy with complete mesocolic excision (CME) and intracorporeal anastomosis? a propensity score-matching study comparison. Surg Endosc 35:2039–2048. https://doi.org/10.1007/s00464-020-07600-w
Magouliotis DE, Baloyiannis I, Mamaloudis I et al (2021) Laparoscopic versus open right colectomy for cancer in the era of complete mesocolic excision with central vascular ligation: pathology and short-term outcomes. J Laparoendosc Adv Surg Tech 31:1303–1308. https://doi.org/10.1089/lap.2020.0508
Zedan A, Elshiekh E, Omar MI et al (2021) Laparoscopic versus open complete mesocolic excision for right colon cancer. Int J Surg Oncol 2021:8859879. https://doi.org/10.1155/2021/8859879
Shin JK, Kim HC, Lee WY et al (2018) Laparoscopic modified mesocolic excision with central vascular ligation in right-sided colon cancer shows better short- and long-term outcomes compared with the open approach in propensity score analysis. Surg Endosc 32:2721–2731. https://doi.org/10.1007/s00464-017-5970-6
Benz S (2013) Survival after complete mesocolic excision (CME) for right sided coloncancer compared to standard surgery. Open Surg J. 7:6–10
Bae SU, Saklani AP, Lim DR et al (2014) Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol 21:2288–2294. https://doi.org/10.1245/s10434-014-3614-9
Chen Z, Sheng Q, Ying X, Chen W (2017) Comparison of laparoscopic versus open complete mesocolic excision in elderly patients with right hemicolon cancer: retrospective analysis of one single cancer. Int J Clin Exp Med 10:5116–5124
Huang J-L, Wei H-B, Fang J et al (2015) Comparison of laparoscopic versus open complete mesocolic excision for right colon cancer. Int J Surg 23:12–17. https://doi.org/10.1016/j.ijsu.2015.08.037
Kim IY, Kim BR, Choi EH, Kim YW (2016) Short-term and oncologic outcomes of laparoscopic and open complete mesocolic excision and central ligation. Int J Surg 27:151–157. https://doi.org/10.1016/j.ijsu.2016.02.001
Sheng Q-S, Pan Z, Chai J et al (2017) Complete mesocolic excision in right hemicolectomy: comparison between hand-assisted laparoscopic and open approaches. Ann Surg Treat Res 92:90–96. https://doi.org/10.4174/astr.2017.92.2.90
Cho MS, Baek SJ, Hur H et al (2015) Modified complete mesocolic excision with central vascular ligation for the treatment of right-sided colon cancer: long-term outcomes and prognostic factors. Ann Surg 261:708–715. https://doi.org/10.1097/SLA.0000000000000831
Benz S, Tam Y, Tannapfel A, Stricker I (2016) The uncinate process first approach: a novel technique for laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 30:1930–1937. https://doi.org/10.1007/s00464-015-4417-1
Feng B, Ling T-L, Lu A-G et al (2014) Completely medial versus hybrid medial approach for laparoscopic complete mesocolic excision in right hemicolon cancer. Surg Endosc 28:477–483. https://doi.org/10.1007/s00464-013-3225-8
Karachun A, Panaiotti L, Chernikovskiy I et al (2020) Short-term outcomes of a multicentre randomized clinical trial comparing D2 versus D3 lymph node dissection for colonic cancer (COLD trial). Br J Surg 107:499–508. https://doi.org/10.1002/bjs.11387
Gao Z, Wang C, Cui Y et al (2020) Efficacy and safety of complete mesocolic excision in patients with colon cancer: three-year results from a prospective, nonrandomized, double-blind, controlled trial. Ann Surg 271:519–526. https://doi.org/10.1097/SLA.0000000000003012
Olsen ASF, Gundestrup AK, Kleif J et al (2021) Accuracy of preoperative staging with multidetector computed tomography in colon cancer. Colorectal Dis 23:680–688. https://doi.org/10.1111/codi.15415
Balciscueta Z, Balciscueta I, Uribe N et al (2021) D3-lymphadenectomy enhances oncological clearance in patients with right colon cancer. Results of a meta-analysis. EJSO 47:1541–1551. https://doi.org/10.1016/j.ejso.2021.02.020
De Simoni O, Barina A, Sommariva A et al (2021) Complete mesocolic excision versus conventional hemicolectomy in patients with right colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36:881–892. https://doi.org/10.1007/s00384-020-03797-3
Anania G, Davies RJ, Bagolini F et al (2021) Right hemicolectomy with complete mesocolic excision is safe, leads to an increased lymph node yield and to increased survival: results of a systematic review and meta-analysis. Tech Coloproctology 25:1099–1113. https://doi.org/10.1007/s10151-021-02471-2
Díaz-Vico T, Fernández-Hevia M, Suárez-Sánchez A et al (2021) Complete mesocolic excision and D3 lymphadenectomy versus conventional colectomy for colon cancer: a systematic review and meta-analysis. Ann Surg Oncol 28:8823–8837. https://doi.org/10.1245/s10434-021-10186-9
Ferri V, Vicente E, Quijano Y et al (2021) Right-side colectomy with complete mesocolic excision vs conventional right-side colectomy in the treatment of colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36:1885–1904. https://doi.org/10.1007/s00384-021-03951-5
Ow ZGW, Sim W, Nistala KRY et al (2021) Comparing complete mesocolic excision versus conventional colectomy for colon cancer: a systematic review and meta-analysis. Eur J Surg Oncol 47:732–737. https://doi.org/10.1016/j.ejso.2020.09.007
Bertelsen CA, Neuenschwander AU, Jansen JE et al (2015) Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 16:161–168. https://doi.org/10.1016/S1470-2045(14)71168-4
Siani LM, Pulica C (2015) Laparoscopic complete mesocolic excision with central vascular ligation in right colon cancer: long-term oncologic outcome between mesocolic and non-mesocolic planes of surgery. Scand J Surg 104:219–226. https://doi.org/10.1177/1457496914557017
Siani LM, Lucchi A, Berti P, Garulli G (2017) Laparoscopic complete mesocolic excision with central vascular ligation in 600 right total mesocolectomies: safety, prognostic factors and oncologic outcome. Am J Surg 214:222–227. https://doi.org/10.1016/j.amjsurg.2016.10.005
Chandramohan A, Mittal R, Dsouza R et al (2022) Prognostic significance of MR identified EMVI, tumour deposits, mesorectal nodes and pelvic side wall disease in locally advanced rectal cancer. Colorectal Dis 24:428–438. https://doi.org/10.1111/codi.16032
Reynolds IS, Fennelly D (2023) Immunotherapy in surgical oncology. Br J Surg 110:3–5. https://doi.org/10.1093/bjs/znac385
Chan DKH, Buczacki SJA (2022) Stage migration-a negative quality indicator in colon cancer management. Colorectal Dis 24:153–154. https://doi.org/10.1111/codi.16091
University of Turin, Italy (2021) Complete mesocolic excision with central vascular ligation in comparison with conventional surgery for the right colon cancer: an Italian randomized trial. clinicaltrials.gov
Funding
Open access funding provided by University of Geneva.
Author information
Authors and Affiliations
Contributions
GDL and JM conceived and designed the study. GDL acquired the data. All authors interpreted the data and contributed to the writing of the draft manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Lange, G., Davies, J., Toso, C. et al. Complete mesocolic excision for right hemicolectomy: an updated systematic review and meta-analysis. Tech Coloproctol 27, 979–993 (2023). https://doi.org/10.1007/s10151-023-02853-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-023-02853-8