Abstract
Purpose
The aim of this study was to evaluate the efficacy and safety of radiofrequency ablation (RFA) in the management of haemorrhoidal disease with 1 year’s follow-up.
Method
This prospective multicentre study assessed RFA (Rafaelo©) in outpatients with grade II–III haemorrhoids. RFA was performed in the operating room under locoregional or general anaesthesia. Primary endpoint was the evolution of a quality-of-life score adapted to the haemorrhoid pathology (HEMO-FISS-QoL) 3 months after surgery. Secondary endpoints were evolution of symptoms (prolapsus, bleeding, pain, itching, anal discomfort), complications, postoperative pain and medical leave.
Results
A total of 129 patients (69% men, median age 49 years) were operated on in 16 French centres. Median HEMO-FISS-QoL score dropped significantly from 17.4/100 to 0/100 (p < 0.0001) at 3 months. At 3 months, the rate of patients reporting bleeding (21% vs. 84%, p < 0.001), prolapse (34% vs. 91.3%, p < 0.001) and anal discomfort (0/10 vs. 5/10, p < 0.0001) decreased significantly. Median medical leave was 4 days [1–14]. Postoperative pain was 4/10, 1/10, 0/10 and 0/10 at weeks 1, 2, 3 and 4. Seven patients (5.4%) were reoperated on by haemorrhoidectomy for relapse, and three for complications. Reported complications were haemorrhage (3), dysuria (3), abscess (2), anal fissure (1), external haemorrhoidal thrombosis (10), pain requiring morphine (11). Degree of satisfaction was high (+ 5 at 3 months on a − 5/+ 5 scale).
Conclusion
RFA is associated with an improvement in quality of life and symptoms with a good safety profile. As expected for minimally invasive surgery, postoperative pain is minor with short medical leave.
Clinical trial registration and date
Clinical trial NCT04229784 (18/01/2020).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haemorrhoidal disease is common. Its prevalence in the general population is estimated at 40% [1, 2]. After failure of other treatments, surgery improves the quality of life of patients [3, 4]. The standard surgical technique in Europe, described by Milligan and Morgan in 1937 [5], is the submucosal resection of the three main haemorrhoidal bundles. In the long-term, such haemorrhoidectomy is the most effective [6]. However, the long and painful postoperative course could be a barrier for patients. In addition, minimally invasive surgeries for haemorrhoids have been developed, such as stapled haemorrhoidopexy [7] and Doppler arterial ligation with or without mucopexy [8]. The concept of minimally invasive surgery is to treat internal haemorrhoids by intervening only above the dentate line, which limits postoperative pain. The use of a radiofrequency current in the treatment of haemorrhoidal disease is an innovative technique. It consists in delivering 4 MHz radiofrequency energy into the haemorrhoidal vascular tissue by means of rigid electrodes. The energy is converted into heat to destroy haemorrhoidal vessels and initiate a process of submucosal fibrosis. Although radiofrequency ablation (RFA) by the Rafaelo© procedure is already used in many European countries, there are only few studies on this minimally invasive technique [9,10,11,12,13,14]. Most of them are retrospective with low numbers of patients and conducted in one or two centres. Therefore, the GREP (Proctology Research Group for the French Society of Proctology, SNFCP) and the CREGG (Hepato-Gastroenterology Cabinets and Groups Reflection Club) decided to evaluate RFA in a prospective French multicentre study with 1 year’s follow-up.
Methods
Study design
This is a multicentre, national, prospective, open and longitudinal study.
Setting
Patients were included in 16 French proctology clinics between January 2019 and March 2020. Recruitment was halted as a result of the pandemic. Patients had a preoperative visit and postoperative visits at 1, 3, 6 and 12 months after surgery.
Participants
The inclusion criteria were age between 18 and 75 years old, and grade II or III haemorrhoidal disease after failure of medico and non-surgical treatment. For patients on anticoagulants or antiplatelet agents (other than aspirin), a short break was recommended in accordance with recommendations [15]. Exclusion criteria were patients with chronic inflammatory disease, anal fissure, anal fistula, external haemorrhoid disease, haematological disease with hemorrhagic risk, patients unable to interrupt anticoagulants or antiplatelet agents, pregnant or breastfeeding women and patients with pacemakers.
Variables
Like Watson et al. [16] in their randomised study, we decided to choose as primary outcome a quality-of-life score rather than a score focusing solely on symptoms. In effect, surgery is mainly chosen to improve quality of life rather than anatomical correction. Therefore, we chose as our main endpoint the improvement of the HEMO-FISS-QoL score 3 months after RFA. This validated score for evaluation of the burden of haemorrhoidal disease [17] is a self-administered questionnaire of 23 questions covering four dimensions: physical disorders, psychology, defecation and sexuality. We analysed the total HEMO-FISS-QoL score as well as each dimension of the score. Scale results range from 0 (low burden) to 100 (very high burden). Secondary criteria were evolution of the HEMO-FISS-QoL score at 1, 6 and 12 months as well as haemorrhoidal symptoms at 1, 3, 6 and 12 months after surgery. Haemorrhoidal symptoms were rated as “present” or “absent” for prolapse, bleeding, pain and pruritus ani. If a symptom was present, it was evaluated by the Goligher scale for prolapse, by the degree of severity for bleeding (minimal, moderate, severe), by a visual analogue scale (VAS) from 0 to 10 for pain and on a scale of 0 to 10 for pruritus ani. Global anal discomfort was rated from 0 to 10. We also evaluated time of hospitalisation and medical leave (or the number of days of limitation of activities for unemployed persons). To limit bias, medical leave certificates were given for 3 days after surgery and extended for periods of 3 days at the patient’s request. We evaluated safety by reporting complication and anal incontinence. Using a self-reported questionnaire, we evaluated maximal and mean postoperative pain on a 0–10 scale every day during the first month and use of analgesics. Patient satisfaction was assessed on an − 5/+ 5 scale for the evolution of haemorrhoidal disease and the degree of satisfaction 1 year after surgery.
Surgery
Surgeons had to have performed at least five procedures before inclusion. The procedure consists in delivering a current of 4 MHz radiofrequency waves by a Rafaelo© generator (CE marking, F Care Systems) at low temperature by microfibre electrodes using a single-use disposable needle (HPR45I probe, CE1304). The procedure is performed under general or locoregional anaesthesia. A perineal block is performed (20 ml of ropivacaine [4]), then 1% xylocaine is injected under the internal haemorrhoidal bundle to lift it from the internal sphincter. The probe is inserted into the haemorrhoidal bundle 1 cm above the dentate line and tilted away from the internal sphincter (Fig. 1). Pulses of 10–20 s (for a maximum of 2000 J per bundle without exceeding a total of 6000 J) are delivered while progressively removing the needle and are stopped at early appearance of whitening around the needle. The haemorrhoidal bundle is then cooled with a compress soaked in ice-cold saline. This process is repeated depending on the patient’s anatomical condition. Total joules and time of procedure were recorded.
Postoperative prescription
Postoperative prescriptions were standardised according to French recommendations and included [4] metronidazole (1.5 g/day) and ketoprofen (200 mg/day) for 7 days; macrogol, suppositories (carrageenan and titanium dioxide) and cream (dexpanthenol) until healing. In case of pain, paracetamol (500 mg), codeine (30 mg) or tramadol (LP 150 mg), if necessary, were prescribed.
Study size
When writing the protocol (2018), we did not have enough data to be able to calculate a study size. As this study was considered as proof of concept, we arbitrarily chose an objective of 150 patients, which seemed achievable in about 1 year of recruitment and enough to evaluate a few associated factors.
Statistical considerations
For qualitative data, this included the number of completed and missing data as well as the frequency and percentage (referring to filled data) for each modality. Proportions were estimated with their exact 95% CIs when appropriate. Data were compared using the chi-squared test or Fisher’s exact test, according to expected values under the assumption of independence. For quantitative data, this included number of filled and missing data, arithmetic mean, standard deviation, median, first and third quartiles, minimum and maximum. Data were compared using the Student or Mann–Whitney–Wilcoxon tests depending on variable distribution.
A Firth’s penalised likelihood multivariate logistic regression was used to measure the probability for a Milligan–Morgan treatment, adjusted for gender, age, indication for surgery, grade of haemorrhoids, HEMO-FISS-QoL score at month 0, anal discomfort at month 0, prolapse and bleeding.
A Firth’s Penalised Likelihood multivariate logistic regression was used to measure the probability of having a mean daily pain of ≥ 6 at least once after surgery and/or level-3 analgesic, adjusted for gender, age, indication for surgery, grade of haemorrhoids, HEMO-FISS-QoL score at month 0, anal discomfort at month 0, prolapse and bleeding.
All comparisons were performed at a statistical significance level set at p < 0.05.
All calculations were made using SAS for Windows (v 9.4; SAS Institute Inc).
Ethics approval statement
The study protocol was validated by the Ile de France Ethics Committee. The MR 001 reference methodology was applied for CNIL (Commission Nationale de l’Informatique et des Libertés) assessment. The protocol design, analysis of results and drafting of the article were carried out independently from sponsors. Written informed consent forms were collected from patients.
The trial was registered as NCT04229784 (18 January 2020).
Results
Characteristics of population and surgery
Table 1 summarises the characteristics of the patients and surgery.
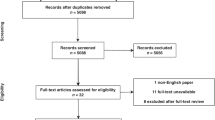
Between January 2019 and March 2020, 129 patients were prospectively included in 16 private or public French centres by 19 proctologists with 1 year’s follow up (Fig. 2). HEMO-FISS-QoL score was available at 3 months for 112 patients (87%). Median patient age was 49 years old; 89 patients (69%) were male. Haemorrhoidal severity according to the Goligher classification was grade III for 70 patients (54.3%) and grade II for 53 patients (41.1%). A few patients with grade I (two patients) or IV (four patients) were included erroneously. The median number of haemorrhoidal bundles per patient was 3 (IQR 3–3). Clopidogrel and direct-acting oral anticoagulants were taken by three and four patients respectively. The main reasons for surgery were bleeding (106 patients, 82.2%) and prolapse (79 patients, 61.2%).
Surgeries were outpatient (122 patients, 97%) or overnight (4 patients, 3%). Median time of surgery was 15 min (IQR 10–25) with a median number of joules delivered of 3925 (IQR 2975–4975). The median number of bundles treated per patient was 3 (IQR 3–4).
Efficiency of radiofrequency surgery
Evolution of HEMO-FISS-QoL score and haemorrhoidal symptoms
With regard to the main assessment criterion in the intention to treat population (N = 129), median HEMO-FISS-QoL scores decreased significantly 3 months after surgery (17.4/100 to 0/100, p < 0.0001) (Table 2 and Fig. 3). The median HEMO-FISS-QoL score remained at 0/100 at 6 and 12 months after surgery. The rate of patients with prolapse decreased significantly at 3 and 12 months (91% vs. 34% and 22%, p < 0.001). At 12 months, only six patients reported grade III haemorrhoids. The rate of patients reporting bleeding significantly decreased to 84% vs. 22% at 3 months and 12 months (p < 0.001). At 12 months, no patient reported severe bleeding and only six reported moderate bleeding. Median global anal discomfort decreased from 5/10 to 0/10 at 3 and 12 months after surgery (p < 0.0001).
In the per protocol population (without grade I and IV patients, N = 123) results are similar to those in the intention to treat population. Median HEMO-FISS-QoL scores significantly decreased 3 months after surgery (from 16.5/100 to 0/100, p < 0.0001).
As one centre accounted for 31% of patients, we checked that there was no centre effect (see supplementary table).
Patient satisfaction
The evolution of haemorrhoidal disease and the degree of satisfaction were, on average, at + 4 and + 5 at 3 and 12 months after surgery on a − 5/+ 5 scale respectively (Table 2). To the question “If it had to be done again, would you agree to be operated on using the same procedure?” 99 patients (86%) answered “yes”. To the question “Would you recommend this operation to relatives?” 94 patients (80%) answered “yes” and 14 patients (12%) answered “perhaps”.
Medical leave time
Medical leave time was a median of 4 days (IQR 1–14; min–max 0–45) and 8 days on average.
Instrumental or surgical re-treatment of haemorrhoids during follow-up
A new infrared treatment of haemorrhoids was carried out in three patients (2.3%). Seven patients (5.7%) were reoperated on during follow-up, all by haemorrhoidectomy using the Milligan–Morgan technique (technique was left to the investigator).
Postoperative pain and analgesics consumption
The median maximum postoperative pain reported was 4/10 (IQR 1–7) during the first week, it decreased to 1/10 (IQR 0–4) the second week, to 0/10 (IQR 0–2) the third week and to 0/10 (IQR 0–1) the fourth week. The median number of level-2 analgesic tablets consumed per day was 0 at weeks 1, 2, 3 and 4. During the first week, 50 patients (42.4%) took at least one level-2 analgesic. Eleven patients (8.5%) had intense pain requiring level-3 analgesics (morphine).
Safety
No perioperative complications were reported. Postoperative complications reported included three postoperative haemorrhages 5, 7 and 8 days after surgery respectively (one required additional surgery), two abscesses (one treated by surgery), four cases of acute urinary retention requiring catheterization, two cases of faecal impaction and one patient reoperated on for anal fissure. Ten patients had postoperative external haemorrhoidal thrombosis, which required level-3 analgesics in three patients. We note that among the seven patients with anticoagulants or clopidogrel, no bleeding was reported. All in all, three patients were reoperated on for complications. Ninety-seven patients (75%) had no complications, without any level-3 analgesics or repeat surgery for recurrence. No significant anal incontinence was reported before or after surgery.
Risks factors of a new haemorrhoidal surgery and having high postoperative pain
We analysed the risk factors for having new haemorrhoidal surgery for recurrence, a hard endpoint of failure. In univariate analysis (Table 3), female gender (OR 5.237 [1.199–30.26]) and presence of pruritus ani at baseline (OR 5.116 [1.167–24.33]) were associated with a risk of reoperation. In multivariate analyses, only pruritus ani at baseline was associated with reoperation (OR 5.8 [1.16–36.4]) (Table 4).
We defined high postoperative pain as corresponding to at least one episode of mean pain level of ≥ 6/10 and/or use of level-3 analgesics (30 patients). In univariate analyses, increased risk of such pain was associated with a global HEMO-FISS-QoL score at baseline > 17/100 (OR 3.476 [1.476–8.844]), a HEMO-FISS-QoL physical disorder dimension > 17/100 (OR 4.482 [1.862–11.90]), a HEMO-FISS-QoL psychology dimension > 17/100 (OR 2.623 [1.138–6.095]), pruritus ani (OR 3.423 [1.383–8.487]) and anal pain (OR 2.455 [1.067–5.689]) at baseline (Table 5). In multivariate analyses, the only two independent associated factors were a global HEMO-FISS-QoL score at baseline > 17/100 and pruritus ani at baseline (OR 3 [1.08–9.12] and 3.6 [1.23–10.89] respectively) (Table 6). When we analysed the dimension of the HEMO-FISS-QoL in a new multivariate analysis, only a HEMO-FISS-QoL physical disorder dimension > 17 and pruritus ani were associated with an increased risk of high postoperative pain with a 3.33 odds ratio [1.20–10.54] and 3.46 [1.158–10.547] respectively.
Discussion
We reported a prospective multicentre evaluation of RFA for the treatment of haemorrhoids in 129 patients with 1 year’s follow-up. RFA is associated with a significant improvement in the HEMO-FISS-QoL score, prolapse, bleeding and global anal discomfort at 3 months, and continued to show a high degree of patient satisfaction at 1 year. As expected from a minimally invasive technique, we observed mild postoperative pain for most patients and short medical leave (median 4 days). However, intense pain requiring level-3 analgesics was observed in 11 patients (8.5%) and patients should be warned of this possibility before surgery. Regarding safety, we observed the usual proctological surgery complications with three patients (2.3%) having to be reoperated on for complications. A specific complication seemed to be painful postoperative external thrombosis (10 patients, or 7.7%). Unexpectedly, having pruritus ani at baseline was the only risk factor found to be associated with reoperation by haemorrhoidectomy in multivariate analysis. This analysis must be interpreted with caution because of the lack of power reflected by the wide confidence intervals observed. One explanation of this observation could be an association of pruritus ani with external haemorrhoidal disease, which is not treated by radiofrequency and therefore a source of reoperation by classic haemorrhoidectomy. Pruritus ani and a baseline HEMO-FISS-QoL score > 17/100 were found to be factors associated with severe postoperative pain in multivariate analysis. Regarding pain, it is known that pruritus leads to central sensitisation and allokinesis or hyperkinesis [18]. Evaluation of the HEMO-FISS-QoL score at baseline, specifically the physical disorders score, may be useful in determining the patients most at risk for postoperative pain.
Other studies on RFA are mostly retrospective and monocentric [9,10,11,12]. Only Schäfer’s team has conducted two prospective studies [13, 14], the most recent of which is bicentric with 98 patients and 2 years’ follow-up [14]. As in our study, RFA was associated with significant symptom reduction and excellent patient satisfaction, low postoperative pain for most patients and on average few days of medical leave. The need for further treatment of haemorrhoids (instrumental or surgical) is a robust indicator of failure. In our study, 7 (5.4%) were reoperated on using Milligan–Morgan haemorrhoidectomy and 3 (2.3%) had infrared treatment. Tolksdorf et al. [14] reported the need for further surgery in 13% of patients (one underwent a stapled haemorrhoidopexy and 12 patients required rubber band ligatures after 2 year follow-up. Tolksdorf et al. [14] treated most of their patients under local anaesthesia while our patients were operated on under general or locoregional anaesthesia. It should be noted that these authors only included patients with a maximum of one or two pathological bundles, whereas in our studies, a median of 3 bundles were treated because the goal was to treat patients with more severe haemorrhoids in one session. In addition, they observed two bleeding episodes per procedure requiring suturing during the intervention [14]. The complication profile found in other studies is similar to our findings.
Our results can be compared with a meta-analysis comparing trans-anal haemorrhoidal dearterialisation with mucopexy (THDm) versus open haemorrhoidectomy [19]. The rate of reoperation for recurrence in our study was close to that of THDm (5.4% and 4.5% respectively) and higher than the rate observed with open haemorrhoidectomy (2.4%). The average medical leave time in our study was lower than with THDm (8 vs. 11 days on average) and considerably lower than that of open haemorrhoidectomy (22 days). Regarding average operating times, RFA is more rapid (15 min) than other techniques (30 min and 23 min for THDm and open haemorrhoidectomy respectively).
The strengths of our study are its prospective and multicentre character, quality of data collection and use of a validated quality of life score as primary assessment criterion. Weaknesses are the lack of comparison with other techniques, a relatively short follow-up interval, as well as few patients on anticoagulants and absence of RFA under local anaesthesia.
For clinicians and policy makers, this study indicates that RFA is safe and effective and should be recognised by the health authorities in order to validate reimbursement. For researchers, this study indicates that there is still potential for surgical innovation for this old pathology.
In conclusion, this is the largest prospective multicentre study on RFA for haemorrhoidal disease involving 129 patients with 1 year follow-up. In patients with grade II–III haemorrhoids without external disease, and after failure of medico-instrumental therapy, this approach provides an excellent efficacy and safety profile that meets the expectations of a minimally invasive technique. Longer-term and randomised studies are needed to confirm our findings. Two clinical trials are currently under way and will compare RFA versus haemorrhoid artery ligation (HAL) Doppler and versus laser.
Data availability
The data that support the findings of this study are available from the corresponding author, [AL], upon reasonable request.
References
Riss S, Weiser FA, Schwameis K et al (2012) The prevalence of hemorrhoids in adults. Int J Colorectal Dis 27(2):215–220
Tournu G, Abramowitz L, Couffignal C et al (2017) Prevalence of anal symptoms in general practice: a prospective study. BMC Fam Pract 18(1):78
MacRae HM, McLeod RS (1995) Comparison of hemorrhoidal treatment modalities. A meta-analysis. Dis Colon Rectum 38(7):687–94
Higuero T, Abramowitz L, Castinel A et al (2016) Guidelines for the treatment of hemorrhoids (short report). J Visc Surg 153(3):213–218
Milligan ETC, Naunton Morgan C, Jones LE, Officer R (1937) Surgical anatomy of the anal canal, and the operative treatment of hæmorrhoids. Lancet 230(5959):1119–1124
Simillis C, Thoukididou SN, Slesser AAP, Rasheed S, Tan E, Tekkis PP (2015) Systematic review and network meta-analysis comparing clinical outcomes and effectiveness of surgical treatments for haemorrhoids. Br J Surg 102(13):1603–1618
Lu Y, Gao R, Liao Z, Hu LH, Li ZS (2010) Meta-analysis of capsule endoscopy in patients diagnosed or suspected with esophageal varices. World J Gastroenterol 15(10):1254–1258
Morinaga K, Hasuda K, Ikeda T (1995) A novel therapy for internal hemorrhoids: ligation of the hemorrhoidal artery with a newly devised instrument (Moricorn) in conjunction with a Doppler flowmeter. Am J Gastroenterol 90(4):610–613
Renshaw S, Eddama MMR, Everson M et al (2019) Radiofrequency ablation for haemorrhoidal disease: description of technique. Tech Coloproctol 23(4):397–398
Hassan S, McGrath D, Barnes R, Middleton S (2021) Radiofrequency ablation (Rafaelo procedure) for the treatment of hemorrhoids: a case series in the United Kingdom. Annal Coloproctol. https://doi.org/10.3393/ac.2021.00276.0039
Didelot JM, Didelot R (2021) Radiofrequency thermocoagulation of haemorrhoidal bundles, an alternative technique for the management of internal haemorrhoids. Int J Colorectal Dis 36(3):601–604
Drissi F, Jean MH, Abet E (2021) Evaluation of the efficacy and morbidity of radiofrequency thermocoagulation in the treatment of hemorrhoidal disease. J Visc Surg 158(5):385–389
Schäfer H, Tolksdorf S, Vivaldi C (2018) Radiofrequency ablation for prolapsing stage III hemorrhoids (Rafaelo® procedure): technique and first clinical results. Coloproctology 40(3):204–210
Tolksdorf S, Tübergen D, Vivaldi C et al (2022) Early and midterm results of radiofrequency ablation (Rafaelo® procedure) for third-degree haemorrhoids: a prospective, two-centre study. Tech Coloproctol 26(6):479–487
HAS. Antiagrégants plaquettaires: prise en compte des risques thrombotique et hémorragique en cas de geste endoscopique chez le coronarien. 2012.
Brown SR, Tiernan JP, Watson AJM et al. Haemorrhoidal artery ligation versus rubber band ligation for the management of symptomatic second-degree and third-degree haemorrhoids (HubBLe): a multicentre, open-label, randomised controlled trial. Lancet. 2016;388(10042):356–64. http://linkinghub.elsevier.com/retrieve/pii/S0140673616305840
Abramowitz L, Bouchard D, Siproudhis L et al (2019) Psychometric properties of a questionnaire (HEMO-FISS-QoL) to evaluate the burden associated with haemorrhoidal disease and anal fissures. Colorectal Dis 21(1):48–58
Misery L (2020) Pruriplastic itch—a novel pathogenic concept in chronic pruritus. Front Med 20(7):615118
Xu L, Chen H, Lin G, Ge Q, Qi H, He X (2016) Transanal hemorrhoidal dearterialization with mucopexy versus open hemorrhoidectomy in the treatment of hemorrhoids: a meta-analysis of randomized control trials. Tech Coloproctol 20(12):825–833
Acknowledgements
The authors would like to thank Ramsay Santé, F Care Systems, YANSYS, ECTEN, IQVIA, HORIANA for their valuable participation in the study.
Funding
Ramsay santé.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LA and AL. The first draft of the manuscript was written by AL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Abramowitz Laurent, Laurain Anne and Dominique Bouchard are consultants for F Care. Aurelien Venara is consultant for Intuitive surgery, Sanofi Aventis, Takeda, Thermofisher. Guillaume Bonnaud is consultant for Abbvie, Alfasigma, Bouchara Recordatti, Celltrion, Ferring, Janssen, Gilead, Medtronic, MSD, Norgine, PFIZER, Roche, Takeda, Tillotts. The other authors declare that they have no conflict of interest.
Ethical approval
The study protocol was validated by the Ile de France Ethics Committee. The MR 001 reference methodology was applied for the CNIL assessment. The protocol design, the analysis of results and the drafting of the article were carried out independently from the study sponsors.
Patient consent
Written informed consent forms were collected from the patients.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laurain, A., Bouchard, D., Rouillon, JM. et al. French multicentre prospective evaluation of radiofrequency ablation in the management of haemorrhoidal disease. Tech Coloproctol 27, 873–883 (2023). https://doi.org/10.1007/s10151-023-02787-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-023-02787-1