Abstract
Background
During ileal pouch-anal anastomosis (IPAA) surgery for ulcerative colitis (UC), rectal dissection can be performed via close rectal dissection (CRD) or in a total mesorectal excision plane (TME). Although CRD should protect autonomic nerve function, this technique may be more challenging than TME. The aim of this study was to compare long-term outcomes of patients undergoing CRD and TME.
Methods
This single-centre retrospective cohort study included consecutive patients who underwent IPAA surgery for UC between January 2002 and October 2017. Primary outcomes were chronic pouch failure (PF) among patients who underwent CRD and TME and the association between CRD and developing chronic PF. Chronic PF was defined as a pouch-related complication occurring ≥ 3 months after primary IPAA surgery requiring redo pouch surgery, pouch excision or permanent defunctioning ileostomy. Secondary outcomes were risk factors and causes for chronic PF. Pouch function and quality of life were assessed via the Pouch dysfunction score and Cleveland global quality of life score.
Results
Out of 289 patients (155 males, median age 37 years [interquartile range 26.5–45.5 years]), 128 underwent CRD. There was a shorter median postoperative follow-up for CRD patients than for TME patients (3.7 vs 10.9 years, p < 0.01). Chronic PF occurred in 6 (4.7%) CRD patients and 20 (12.4%) TME patients. The failure-free pouch survival rate 3 years after IPAA surgery was comparable among CRD and TME patients (96.1% vs. 93.5%, p = 0.5). CRD was a no predictor for developing chronic PF on univariate analyses (HR 0.7 CI-95 0.3–2.0, p = 0.54). A lower proportion of CRD patients developed chronic PF due to a septic cause (1% vs 6%, p = 0.03).
Conclusions
Although differences in chronic PF among CRD and TME patients were not observed, a trend toward TME patients developing chronic pelvic sepsis was detected. Surgeons may consider performing CRD during IPAA surgery for UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restorative proctocolectomy with subsequent ileal pouch-anal anastomosis (IPAA) is a treatment option for patients with refractory ulcerative colitis (UC), familial adenomatous polyposis (FAP) and UC-related dysplasia. Proctectomy with IPAA can be performed at the time of colectomy (1- or 2-stage surgery) or following subtotal colectomy in a second stage completion proctectomy (modified 2- or 3-stage surgery). Both IPAA construction and (completion) proctectomy are associated with serious short- and long-term postoperative morbidity, such as anastomotic leakage which occurs in 10–20% of patients, and chronic pouch failure (PF) which occurs in 5–10% of patients [1,2,3,4]. Innovations in surgical techniques have been developed to improve short- and long-term outcomes after IPAA surgery [5,6,7]. One of the many surgical challenges during ileoanal pouch surgery is the dissection of the rectum. Dissection in the total mesorectal excision (TME) plane, similar to a dissection for cancer, should follow an avascular embryological plane, but autonomic nerves are at risk. Damage may result in sexual and/or urinary dysfunction [8]. In our institution, surgeons performed a posterior TME, preserving the anterolateral mesorectum as per European Crohn’s and Colitis organisation (ECCO) recommendations until 2007 [9]. A technically more challenging, complete close rectal dissection (CRD) was introduced in 2007 to spare the autonomic nerves. During CRD, dissection is performed through a non-anatomical perimuscular plane, well away from the autonomic nerves. This leaves a smaller dead space in the pelvis postoperatively, which may reduce postoperative septic collections in the pelvis [10, 11]. However, there is a potential risk of haemorrhage from the mesorectal vessels.
These rectal dissection techniques have been compared in a randomized controlled trial assessing short-term postoperative morbidity [12]. Results demonstrated that CRD led to a lower severe complication rate and better short-term quality of life when compared to TME. CRD has been performed as default technique for UC in our centre ever since. However, to date, long-term data on quality of life, pouch function and chronic PF among patients who underwent TME and CRD have been reported. The aim of this study was to assess chronic PF rates and long-term failure-free pouch survival among patients who underwent (completion) proctectomy and IPAA surgery via CRD and TME in a tertiary referral centre for pouch surgery.
Materials and methods
Design and patients
In this retrospective cohort study, all consecutive adult patients who underwent IPAA for UC in the Amsterdam University Medical Centers (UMC), location Academic Medical Center (AMC), between January 2002 and October 2017 were included. This is a tertiary referral centre with experienced inflammatory bowel disease (IBD) surgeons. Patient data were stored in a database constructed by four of the authors (MR, DJ, EW and EB). Patients who underwent IPAA surgery with a histopathologic diagnosis or suspicion of FAP, Crohn's disease or IBD-undetermined in the colectomy resection specimen, and patients undergoing redo-pouch surgery were excluded. Patients with unspecified rectal dissection types were also excluded. The medical ethics board have assessed and deemed review unnecessary for this study. All participants provided informed consent via opt-out procedure. Reporting of the data adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
Procedures
IPAA
All IPAA procedures were performed by experienced colorectal surgeons. Surgical creation of an IPAA was performed during initial proctocolectomy (1- or 2-stage IPAA surgery), or at the time of completion proctectomy (modified 2- or 3-stage surgery). A 10 cm J (or modified J) ileal pouch was created via transanal or transabdominal (by laparotomy or laparoscopy) approach, or a combined approach. A pouch-anal anastomosis was routinely constructed with a circular stapler. A defunctioning ileostomy was created selectively, based on intraoperative findings (e.g. traction on pouch-anal anastomosis, positive air-leak test).
Proctectomy types
Prior to the introduction of CRD as a dissection technique, a TME or ‘sub-TME’ was performed. The sub-TME was defined as an anterior rectal dissection performed on the outer muscle layer of the distal rectum with posterolateral mesorectal dissection. Subsequent to 2007, a CRD was routinely performed, leaving the mesorectum in situ by working in the non-anatomical perimuscular plane, sparing the superior rectal artery (Fig. 1) [11].
Resection specimens and schematic transversal resection margins for CRD (green), TME (red) and sub-TME (blue) dissection [11]. TME total mesorectal excision, CRD close rectal dissection, Ant anterior side of rectum, R rectum, MR mesorectal fat
Outcomes
Outcomes were compared for patients undergoing TME (including sub-TME) and CRD. Primary outcomes of this study were chronic PF and failure-free pouch survival at end of follow-up and 3 years after IPAA surgery. Secondary outcomes were risk factors and causes for chronic PF. Pouch function and quality of life were assessed via the Pouch dysfunction score (PDS) [13] (Appendix A) and Cleveland global quality of life (CGQL) score [14] (Appendix B).
Definitions
Chronic PF was defined as a long-term (≥ 3 months) pouch-related complication (including pouch dysfunction) following initial IPAA surgery requiring pouch redo surgery or excision regardless of the surgical indication and functional outcome of the pouch (with or without permanent diversion by ileostomy). Chronic PF did not include redo-pouch surgery performed within 3 months postoperatively for acute postoperative complications. Follow-up was calculated from the first day of faecal stream through the pouch until the date of the last follow-up visit. In the current study, potential postoperative risk factors for chronic PF were assessed. Type of dissection, postoperative pelvic sepsis, and pouch fistula were included in the analyses. Pelvic sepsis included acute anastomotic leakage and/or chronic pelvic sepsis. A fistula that occurred after IPAA surgery originating from anywhere in the pouch was defined as a postoperative pouch fistula. Both pelvic sepsis and postoperative pouch fistula were diagnosed on postoperative cross-sectional imaging (computed tomography scan or magnetic resonance imaging).
Statistical analysis
Categorical outcomes were expressed as percentage. Continuous levels of secondary outcomes showing were expressed as mean with standard deviation (SD) or median with interquartile range (IQR), according to the distribution. To compare categorical data, the Chi-square test was used for statistical significance. For comparing continuous data with normal distribution, the independent samples T-test was used. In case continuous data were not normally distributed, the Mann–Whitney U test was used to compare continuous levels. Uni- and multivariate Cox-regression analysis was used for analysis of failure-free pouch survival. CRD, pelvic sepsis and pouch fistula were included in the multivariate Cox-regression model. Pelvic sepsis and pouch fistula are established independent risk factors for chronic PF according to literature [4]. The final model was established by stepwise manual backward selection (p < 0.05 to stay in the model). Outcomes of the multivariate Cox-regression analysis are presented as hazard ratios (HR) with 95% confidence intervals (CI). Survival analyses were performed via Kaplan–Meier analyses with log-rank as comparison test. Levels with missing data exceeding 10% of all cases were excluded from Cox-regression analyses. Agreement between fistula and pelvic sepsis was calculated by the Kappa measure of agreement test; agreement was slight, fair, moderate, substantial or perfect for 0.0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80 and 0.81–1.00, respectively [15]. Data were analysed through complete case analyses. Differences were considered statistically significant if two-sided p values were below 0.05. Statistical analyses were performed using SPSS (IBM Corp., Armonk, NY, USA).
Results
Demographics
Ileoanal pouches were created in 341 UC patients between January 2002 and October 2017. A total of 52 patients were excluded from this study as there was a diagnosis other than UC (n = 29), the rectal dissection type remained unspecified (n = 20) or the IPAA never became functional (n = 3). The median age of the 289 included patients was 37.0 years (IQR 26.5–45.5 years), and 155 (53.6%) were male. Overall, 128 (44.3%) underwent proctectomy via CRD, and 161 (55.7%) patients underwent (sub)TME. Within the TME cohort, 54 (33.5%) patients underwent a sub-TME. Median age and sex were comparable among CRD and (sub)TME (35.0, IQR 25.8–45.3 vs. 38.0 IQR 27.5–46.0 and 69 (53.9%) vs 86 (53.4%) male, p = 0.17 and 0.93, respectively). The majority of the patients who underwent IPAA underwent proctectomy via a transabdominal approach (78.2%). A larger proportion of patients who underwent proctectomy via a CRD underwent IPAA surgery via a modified 2-stage IPAA surgery (78.9% vs. 41.0%, p < 0.01) and transanal proctectomy (46.1% vs. 1.2%, p < 0.01) when compared to patients who underwent (sub)TME. Further baseline characteristics are shown in Table 1.
Long-term outcomes after IPAA surgery
After a median follow-up of 7.2 (IQR 3.4–11.6) years, 26 out of 289 patients (9.0%) developed chronic PF. The median follow-up for patients with CRD was significantly shorter when compared to (sub)TME (3.7 vs. 10.9 years, p < 0.01). Chronic PF occurred in a smaller proportion of patients who underwent CRD when compared to patients who underwent (sub)TME (4.7% vs 12.4%, p = 0.02). Three (1.0%) patients were excluded from the survival analyses as they were lost to follow-up. Overall, mean chronic failure-free pouch survival was 17 (CI 16.2–17.8) years, and was comparable between patients who underwent CRD and (sub)TME (14.0 vs 16.8) years, p = 0.49). Failure-free pouch survival rates after 36 months were 96.1% for patients with a CRD dissection and 94.2% for patients with a (sub)TME dissection. Survival curves are shown in Fig. 2.
Causes of chronic PF
Out of the six patients who developed chronic PF requiring surgery after CRD, one was a result of a chronic sinus. One patient developed chronic refractory pouchitis, and two patients developed outlet complications. Two patients underwent permanent defunctioning for dysfunction caused by high defecation frequency (n = 1) and incontinence (n = 1).
Out of 20 patients with chronic PF after (sub)TME, 10 had septic complications causing failure. Four patients developed pouchitis, 1 patient had inlet complications and 3 patients developed outlet complications. Two patients underwent permanent defunctioning for severe dysfunction due to incontinence (n = 1) and dysfunction after a radiation induced ulcer for prostate cancer (n = 1).
Figure 3 shows causes of chronic PF per rectal dissection technique. A significantly lower proportion of chronic PF caused by septic complications was found in CRD patients when compared to (sub)TME (0.8% vs 6.2%, p = 0.03). The septic failure-free survival was 100% in the CRD group compared to 96.6% in the (sub)TME group (log rank p = 0.2, Fig. 4).
Risk factors for chronic PF
In this cohort, 42 patients (14.5%) developed pelvic sepsis. A chronic sinus not preceded by an established acute postoperative anastomotic leakage was diagnosed in 5 (1.7%) patients. Acute anastomotic leakage occurring within 90 days postoperatively was diagnosed in 37 (12.8%) patients, 19 14.8% in the CRD group and 18 (11.4%) in the (sub)TME group (p = 0.39). As of 2006, Endovac therapy was applied in 19 patients with acute anastomotic leakage, and combined with early surgical closure (EVASC) in 15 of these patients [16]. None of the patients who underwent CRD surgery followed by EVASC-treated acute anastomotic leakage developed chronic PF caused by septic complications (n = 12). Two out of three (sub)TME patients developed chronic PF caused by septic complications after EVASC therapy for anastomotic leakage. Moreover, 2 out of 4 (sub)TME patients with Endovac treatment alone developed chronic pelvic sepsis. Overall, 29 patients (10.0%) developed a pouch-related fistula. Univariate analyses revealed pelvic sepsis and pouch fistula as risk factors for developing chronic PF (HR 6.3, CI 95% 2.9–13.7, p < 0.001 and HR 7.1, CI 95% 3.3–15.4, p < 0.001, respectively). CRD was not a risk factor for developing chronic PF (HR 0.7 CI 0.3–1.9, p = 0.48). In multivariate analyses including pelvic sepsis, fistula and CRD, pelvic sepsis and fistula were independent risk factors for developing chronic PF (HR 3.3, p = 0.01 and HR 4.3, p < 0.01). A slight agreement was observed between pelvic sepsis and fistula (κ: 0.40). Results are shown in Table 2.
Pouch function
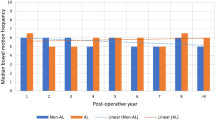
After excluding 26 patients who developed chronic PF, the response rate for the pouch function questionnaires was 215/263 (81.7%). Follow-up was shorter for CRD patients compared to (sub)TME (31.5 months, IQR 16.0–51.3 vs. 126 months, IQR 78.8–150.8, p < 0.01). According to the PDS (Appendix A), 145 patients (67.4%) had ‘none to minor’ pouch dysfunction, and 70 patients (32.6%) had ‘some to major’ pouch dysfunction. When comparing 110 patients who underwent CRD with 106 patients who underwent (sub)TME, 32 (29.1%) of CRD patients had some/major dysfunction, compared to 38 (36.2%) of TME patients (p = 0.27). A lower proportion of CRD patients reported a defecation frequency exceeding ten times per 24 h, when compared to TME patients (21, 19.4% vs 32, 30.1%, p = 0.06). No difference in the continuous total PDS was reported between patients who underwent CRD and TME (1.5, IQR 0.4–2.5 vs. 1.5, IQR 0.5–2.9, p = 0.26) (see Fig. 5). Quality of life according to the CGQL (Appendix B) was similar among patients who underwent CRD and TME (0.73 IQR 0.67–0.83 vs. 0.73 IQR 0.63–0.80, p = 0.32).
Discussion
We found no significant differences in long-term outcomes between patients who had CRD during IPAA for UC and those who had (sub)TME. Failure-free pouch survival and functional outcomes were similar among cohorts and CRD was not associated with developing chronic PF. After a median follow-up of 7.2 years, an overall chronic PF rate of 9.0% was found in this study, which is in line with available literature [4]. Although chronic PF rates of CRD patients were significantly lower when compared to (sub)TME (4.7% vs. 12.4%, p < 0.01), this finding was confounded by a significantly shorter postoperative follow-up for patients who underwent CRD surgery (3.7 vs. 10.9 years, p < 0.01). No significant difference remained after correction for length of follow-up in survival analyses (p = 0.49). Pelvic sepsis and fistula were independent risk factors for chronic PF in the current study, which corresponds with the literature [4]. A larger proportion of patients who underwent CRD had a proctectomy via transanal minimally invasive surgery combined with a laparoscopic abdominal approach. Furthermore, a larger proportion of patients who underwent CRD underwent modified two -stage pouch surgery. These differences relate to the change in approach adopted by our unit over the period of the study. In the present study, there was a lower proportion of chronic PF secondary to a septic cause in CRD patients when compared to patients who underwent (sub)TME (0.8% vs. 6.2%). This is consistent with previous studies and perhaps supports the hypothesis that mesorectal fat preserved during proctectomy has a protective role against pelvic sepsis; less dead space remains in the pelvis postoperatively and both the lymphatic and nervous system are maintained [10, 17]. However, a potential confounder is the introduction of EVASC for acute anastomotic leakage in 2010, which may have also reduced pouch failure due to chronic pelvic sepsis. Nevertheless, 2 out of 3 (sub)TME patients with EVASC-treated acute anastomotic leakage developed chronic pelvic sepsis, whereas none of the 12 CRD patients did. Another potential confounder to our results is the introduction of a more intense postoperative diagnostic protocol introduced during the study period. Whilst the acute postoperative anastomotic leakage rates were similar among CRD and (sub)TME patients, we hypothesize that the diagnosis of acute postoperative leakage after (sub)TME may have been underestimated, resulting in an increased proportion of chronic septic complications. A chronic sinus generally does not appear de novo after absence of acute postoperative complications, as it is most likely a product of (un)diagnosed acute postoperative anastomotic leakage [5, 18, 19]. Undiagnosed postoperative leakage may have been less common after CRD surgery because this intensive follow up protocol was introduced around the same time. The increased incidence of PF caused by septic complications after (sub)TME surgery could therefore, to a lesser extent, be due to the difference in postoperative follow-up.
To the best of our knowledge, this is the first study assessing long-term outcomes after CRD and (sub)TME proctectomy during IPAA surgery. This is a large cohort of 289 patients with a median follow-up period exceeding seven years. Although comorbidities were not reported, ASA scores representing overall preoperative condition were collected and similar among cohorts. Therefore, this study attempted to take potential confounding risk factors for chronic PF into account. Nevertheless the study has some limitations in addition to the potential innovation confounders discussed above. Although the retrospective nature of this study may have resulted in missing data on baseline characteristics and follow-up, pouch complications were, by default, diagnosed and (surgically) treated in our referral centre. Therefore, the follow-up is likely to be complete. We assessed pouch function and quality of life via validated questionnaires. Pouch function and quality of life were comparable among cohorts, with a slightly lower proportion of CRD patients who experienced a high frequency of defecation. However, conclusions cannot be adequately drawn based on these results due to the difference in follow-up time. One potential benefit of a CRD approach is the hypothesis that this technique might spare the autonomic nerves involved in sexual and urinary functions. Earlier studies have not reported significant differences in sexual function among patients who underwent CRD and TME [22, 23]. In the current study, we did not record sexual and urinary function, as these were not routinely assessed during postoperative outpatient visits. Furthermore, the shorter postoperative follow-up period in CRD patients preclude adequate comparison of data on sexual and urinary function. Potential major confounding factors in terms of surgical technique (e.g. number of stages, laparoscopic or open abdominal approach) were not included in multivariate analyses due to a limited number of chronic PF events (n = 26). Two risk factors for chronic PF (pelvic sepsis and pouch fistula), previously established by a meta-analysis, were included in our multivariate analyses. Although pouch fistula and pelvic sepsis were both independent risk factors for chronic PF, these complications occasionally occur concurrently and a causal relation between anastomotic leakage and developing fistula may reasonably be inferred. However, only a slight level of agreement between these variables was found. Moreover, this correlation should not have interfered with (multivariable) analyses since CRD was not associated with chronic PF in analyses.
Conclusions
Chronic failure-free pouch survival rates were comparable among cohorts and CRD was not an (independent) risk factor for chronic PF. Patients who underwent CRD had a lower rate of chronic PF caused by pelvic sepsis, possibly due to decreased postoperative dead space in the pelvis after CRD surgery and implementation of EVASC. Although future studies evaluating long-term pouch function and quality of life are warranted, results of this study demonstrate at least similar long-term outcomes after CRD when compared to (sub)TME.
References
Widmar M, Munger JA, Mui A et al (2019) Diverted versus undiverted restorative proctocolectomy for chronic ulcerative colitis: an analysis of long-term outcomes after pouch leak short title: outcomes after pouch leak. Int J Colorectal Dis 34(4):691–697. https://doi.org/10.1007/s00384-019-03240-2 (published Online First: Epub Date)
Sahami S, Bartels SA, D’Hoore A et al (2016) A multicentre evaluation of risk factors for anastomotic leakage after restorative proctocolectomy with ileal pouch-anal anastomosis for inflammatory bowel disease. J Crohns Colitis 10(7):773–778. https://doi.org/10.1093/ecco-jcc/jjv170 (published Online First: Epub Date)
Sossenheimer PH, Glick LR, Dachman AH et al (2019) Abnormal pouchogram predicts pouch failure even in asymptomatic patients. Dis Colon Rectum 62(4):463–469. https://doi.org/10.1097/DCR.0000000000001285 (published Online First: Epub Date)
Heuthorst L, Wasmann KATGM, Reijntjes MA, Hompes R, Buskens CJ, Bemelman WA (2021) Ileal Pouch-anal anastomosis complications and pouch failure: a systematic review and meta-analysis. Ann Surg Open 2(2):e074. https://doi.org/10.1097/as9.0000000000000074 (published Online First: Epub Date)
de Buck van Overstraeten A, Mark-Christensen A, Wasmann KA et al (2017) Transanal versus transabdominal minimally invasive (Completion) proctectomy with ileal pouch-anal anastomosis in ulcerative colitis: a comparative study. Ann Surg 266(5):878–883. https://doi.org/10.1097/SLA.0000000000002395 (published Online First: Epub Date)
Wasmann KA, Reijntjes MA, Stellingwerf ME et al (2019) Endo-sponge assisted early surgical closure of ileal pouch-anal anastomotic leakage preserves long-term function: a cohort study. J Crohns Colitis 13(12):1537–1545. https://doi.org/10.1093/ecco-jcc/jjz093 (published Online First: Epub Date)
Gardenbroek TJ, Musters GD, Buskens CJ et al (2015) Early reconstruction of the leaking ileal pouch-anal anastomosis: a novel solution to an old problem. Colorectal Dis 17(5):426–432. https://doi.org/10.1111/codi.12867 (published Online First: Epub Date)
Sartori CA, Sartori A, Vigna S, Occhipinti R, Baiocchi GL (2011) Urinary and sexual disorders after laparoscopic TME for rectal cancer in males. J Gastrointest Surg 15(4):637–643. https://doi.org/10.1007/s11605-011-1459-0 (published Online First: Epub Date)
Oresland T, Bemelman WA, Sampietro GM et al (2015) European evidence based consensus on surgery for ulcerative colitis. J Crohns Colitis 9(1):4–25. https://doi.org/10.1016/j.crohns.2014.08.012 (published Online First: Epub Date)
Nally DM, Kavanagh DO, Winter DC (2019) Close rectal dissection in benign diseases of the rectum: a review. Surgeon 17(2):119–126. https://doi.org/10.1016/j.surge.2018.06.002 (published Online First: Epub Date)
Garcia-Granero A, Pellino G, Fletcher-Sanfeliu D et al (2022) “Near-TME”: proposed standardisation of the technique for proctectomy in male patients with ulcerative colitis. Tech Coloproctol 26(3):217–226. https://doi.org/10.1007/s10151-022-02579-z (published Online First: Epub Date)
Bartels SA, Gardenbroek TJ, Aarts M et al (2015) Short-term morbidity and quality of life from a randomized clinical trial of close rectal dissection and total mesorectal excision in ileal pouch-anal anastomosis. Br J Surg 102(3):281–287. https://doi.org/10.1002/bjs.9701 (published Online First: Epub Date)
Brandsborg S, Nicholls RJ, Mortensen LS, Laurberg S (2013) Restorative proctocolectomy for ulcerative colitis: development and validation of a new scoring system for pouch dysfunction and quality of life. Colorectal Dis 15(12):e719–e725. https://doi.org/10.1111/codi.12425 (published Online First: Epub Date)
Kiran RP, Delaney CP, Senagore AJ et al (2003) Prospective assessment of Cleveland Global Quality of Life (CGQL) as a novel marker of quality of life and disease activity in Crohn’s disease. Am J Gastroenterol 98(8):1783–1789. https://doi.org/10.1111/j.1572-0241.2003.07592.x (published Online First: Epub Date)
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW (2008) Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 22(8):1818–1825. https://doi.org/10.1007/s00464-007-9706-x (published Online First: Epub Date)
Rink AD, Radinski I, Vestweber KH (2009) Does mesorectal preservation protect the ileoanal anastomosis after restorative proctocolectomy? J Gastrointest Surg 13(1):120–128. https://doi.org/10.1007/s11605-008-0665-x (published Online First: Epub Date)
Lightner AL, Dattani S, Dozois EJ, Moncrief SB, Pemberton JH, Mathis KL (2017) Pouch excision: indications and outcomes. Colorectal Dis 19(10):912–916. https://doi.org/10.1111/codi.13673 (published Online First: Epub Date)
Mark-Christensen A, Erichsen R, Brandsborg S et al (2018) Pouch failures following ileal pouch-anal anastomosis for ulcerative colitis. Colorectal Dis 20(1):44–52. https://doi.org/10.1111/codi.13802 (published Online First: Epub Date)
Manilich E, Remzi FH, Fazio VW, Church JM, Kiran RP (2012) Prognostic modeling of preoperative risk factors of pouch failure. Dis Colon Rectum 55(4):393–399. https://doi.org/10.1097/DCR.0b013e3182452594 (published Online First: Epub Date)
Sahami S, Bartels SA, D’Hoore A et al (2017) External validation of a prognostic model of preoperative risk factors for failure of restorative proctocolectomy. Colorectal Dis 19(2):181–187. https://doi.org/10.1111/codi.13414 (published Online First: Epub Date)
Lindsey I, George BD, Kettlewell MG, Mortensen NJ (2001) Impotence after mesorectal and close rectal dissection for inflammatory bowel disease. Dis Colon Rectum 44(6):831–835. https://doi.org/10.1007/BF02234703 (published Online First: Epub Date)
Hicks CW, Hodin RA, Savitt L, Bordeianou L (2014) Does intramesorectal excision for ulcerative colitis impact bowel and sexual function when compared with total mesorectal excision? Am J Surg 208(4):499-504e44. https://doi.org/10.1016/j.amjsurg.2014.05.012 (published Online First: Epub Date)
Acknowledgements
M.A. Reijntjes and D.C. de Jong contributed equally to this manuscript and therefore share fist authorship. No writing assistance was used. No funding was received for conduction of this study. This manuscript has not been previously published and the manuscript is not under consideration elsewhere. Bonafide, qualified, scientific and medical researchers may request access to the identified study data. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data access.
Funding
None.
Author information
Authors and Affiliations
Contributions
MR, DJ, MD, WB, RH, CB, GD: contributed to the conception and design of the study. MR, DJ, SB, EW, EB: contributed to the acquisition of data, analysis and interpretation of data and drafting of the article. MR, DJ, SB, EW, EB, RH, CB, GD, MD, WB: critically revised the article for important intellectual content. MR, DJ, SB, EW, EB, RH, CB, GD, MD, WB: approved the final version of the article for submission.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Ethical approval
Reporting of the data adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
Informed consent
All participants provided informed consent via opt-out procedure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A. Pouch dysfunction score

Appendix B. Cleveland global quality of life questionnaire

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reijntjes, M.A., de Jong, D.C., Bartels, S. et al. Long-term outcomes after close rectal dissection and total mesorectal excision in ileal pouch-anal anastomosis for ulcerative colitis. Tech Coloproctol 27, 297–307 (2023). https://doi.org/10.1007/s10151-022-02713-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-022-02713-x