Abstract
Background
Formation of a defunctioning loop ileostomy is common after mid and low rectal resection. Historically, they were reversed between 3 and 6 months after initial resection. Recently, earlier closure (< 14 days) has been suggested by some current randomised controlled trials. The aim of this study was to investigate the effect of early stoma closure on surgical and patient outcomes.
Methods
A systematic review of the current randomised controlled trial literature comparing early and standard ileostomy closure after rectal surgery was performed. Specifically, we examined surgical outcomes including; morbidity, mortality and quality of life.
Results
Six studies met the predefined criteria and were included in our analysis. 275 patients underwent early stoma closure compared with 259 patients having standard closure. Overall morbidity was similar between both groups (25.5% vs. 21.6%) (OR, 1.47; 95% CI 0.75–2.87). However, there tended to be more reoperations (8.4 vs. 4.2%) (OR, 2.02, 95% CI 0.99–4.14) and small bowel obstructions/postoperative ileus (9.3% vs. 4.4%) (OR 0.44, 95% CI 0.22–0.90) in the early closure group, but no difference across the other domains.
Conclusions
Early closure appears to be a feasible in highly selective cases after good perioperative counselling and shared decision-making. Further research on quality of life outcomes and long term benefits is necessary to help define which patients are suitable candidates for early closure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anastomotic leakage is a major complication following rectal surgery, occurring in 3–28% of lower anterior resections [1, 2]. Over the years, the evolution of surgical techniques and technology has facilitated lower pelvic anastomosis, which inherently comes with a higher risk of anastomotic leak [3]. A defunctioning loop ileostomy is often created to divert bowel contents away from the site of anastomosis, thereby reducing the need for re-operation/intervention in presence of an anastomotic leak [4, 5]. The formation of a diverting stoma does not alleviate the risk of anastomotic leak. However, it substantially reduces the morbidity and mortality if one occurs [6].
Ileostomy reversal is generally performed 3–6 months after initial surgery. This may be delayed further if the patient is receiving adjuvant chemotherapy [2]. Unfortunately, while this is generally regarded as the optimal timing of reversal, it is not routine in many healthcare systems. The National Bowel Cancer Audit (NBOCA) annual report 2021 demonstrated that approximately 30% of patients with a diverting ileostomy had not had their stoma reversed 18 months after their initial surgery, an outcome likely exacerbated by the effects of the COVID-19 pandemic [7]. This extended period predisposes approximately 10–30% of patients to stoma-associated morbidity which has been shown to negatively impact quality of life [5]. Delays in these ‘routine’ surgeries have undoubtedly had an effect on these long-term complications and it could, therefore, be argued that an increased uptake of early closure has the potential to alleviate this morbidity [8]. The prolonged presence of an ileostomy can precipitate multiple stoma-related complications, such as prolapse, parastomal hernia, mechanical ileus or dehydration and subsequent kidney injury [9]. These complications may result in readmission to hospital, which in turn increases costs and the burden on the healthcare system.
Low anterior resection syndrome (LARS) consists of a group of symptoms of bowel dysfunction after restorative rectal surgery including faecal urgency, difficulty emptying and incontinence [10] The incidence of LARS increases in patients with a low anastomosis, or in those whom had neoadjuvant chemoradiotherapy [11, 12]. Recently, it has been proposed that those with an extended duration of diversion (ileostomy) following rectal resection have a higher incidence of LARS and an early reversal may help to prevent this [10]. A recent meta-analysis of 11 studies demonstrated that ileostomy reversal within 6 months of initial surgery protects against LARS, while reversal after 1 year was associated with a higher risk of major LARS [13].
While there remains no clear international consensus on the optimal timing of reversal, some surgeons now advocate an early ileostomy closure (EC) within 14 days following primary resection [12, 14]. EC reduces the amount of time a patient lives with an ileostomy as well as costs related to ostomy care [9]. Some postulate that it is associated with improved functional outcomes [10]. While certain studies advocate EC of ileostomies, others have found higher rates of complications, with one trial being terminated early due to higher morbidity in the EC group [12].
The aim of our study was to assess the randomised controlled trials (RCTs) on early vs. late ileostomy closure in the literature and compare outcomes in the EC vs. standard closure (SC) groups. Specifically we examined the success of EC in terms of morbidity, mortality, cost, readmission, length of stay and functional outcomes.
Materials and methods
Study design and reporting guidelines
This study is a systematic review of randomised controlled trials and follows Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guidelines.
Search strategy
The following databases were searched as part of the systematic review in November 2021: Medline, EMBASE and Web of Science. The following search terms were used: “early*”, “standard*”, “late*”, “closure*”, “reversal*”, “ileostomy*”. The symbol “*” was used to allow variations on a word stem to be included in the search results. Furthermore, the following MeSH (medical subject headings) were used: ((((early[MeSH]) OR (late[MeSH])) OR (standard[MeSH])))) AND ((reversal[MeSH])) OR (closure[MeSH])) AND ((ileostomy[MeSH])) OR (stoma[MeSH])) The last search date was December 12rh 2021. A grey literature was also performed to further identify other eligible studies.
Inclusion criteria
Studies in English assessing the outcomes of early vs. late ileostomy reversal were assessed for eligibility based on the following inclusion criteria:
-
i.
Study design:
-
a.
Randomised controlled trials
-
a.
-
ii.
Participants:
-
a.
Patients undergoing rectal surgery, regardless of primary indication
-
b.
Formation of defunctioning ileostomy
-
a.
-
iii.
Intervention:
-
a.
Comparison of early vs. late ileostomy reversal
-
a.
-
iv.
Outcomes:
-
a.
Primary: Quantitative measure of overall morbidity
-
b.
Secondary: Quantitative measure of severe morbidity, mortality, cost, readmission, length of stay and functional outcomes
-
a.
Exclusion criteria
-
i.
Study design:
-
a.
Non-randomised controlled trials
-
a.
-
ii.
Participants:
-
a.
Patients undergoing non-rectal surgery
-
a.
-
iii.
Intervention:
-
a.
No comparison of early vs. late ileostomy reversal
-
b.
Reversal of colostomy
-
a.
-
iv.
Outcome:
-
a.
Qualitative measures only
-
a.
Outcomes of interest
-
i.
Primary outcome: Overall morbidity
-
a.
The primary aim of this study was to quantitatively assess the overall morbidity, as defined by the Clavien–Dindo classification, of both early and late reversal of ileostomy.
-
a.
-
ii.
Secondary outcome:
-
a.
The secondary aims of this study were to quantitatively assess major morbidity (Clavien Dindo > 2), mortality, readmission, cost, length of stay, quality of life, length of procedure and time to chemotherapy.
-
a.
Study selection, data extraction and critical appraisal
A database was created using the reference managing software EndNote X9™. Two researchers (NOS, HT) reviewed outputs from the searches independently of each other.
Initially, duplicates were removed. Study titles were then screened and assessed for potential relevance. The abstracts of selected studies were then read and assessed for eligibility for inclusion, based on the inclusion/exclusion criteria detailed above. Rejected studies were grouped together in the database by their reason for exclusion. The full texts of the abstracts deemed eligible for inclusion were then further analysed using the same criteria. Conflicts between the two reviewers were resolved following an open discussion and final decision by the senior author (MEK).
To extract and store data efficiently, the Cochrane Collaboration screening and data extraction tool, Covidence, was used. Data was collected by two reviewers independently, using the following headings; study details, study design, population, intervention, comparison groups and outcomes. Conflicts between the two reviewers were resolved following an open discussion and final decision by the senior author.
A critical appraisal of the methodological quality and risk of bias of the included studies was performed. The Cochrane Collaboration’s tool for assessing risk of bias was used [15]. This assessment tool grades each study as being high, low, or unclear risk of bias across six categories. The critical appraisal was completed by two reviewers (NOS, HT) independently. Furthermore, the certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) tool for grading quality of evidence [16].
Statistical analysis
Statistical analysis was performed using Revman Statistical Software (Ver. 5 Copenhagen, Denmark). Binary outcome data were reported as Odd ratios (OR) and 95% confidence interval (95% CI) were estimated using the Mantel–Haenszel method. For continuous data, mean differences and 95% CI were estimated using inverse variance weighting. Outcome measures (mean + standard deviation and median + interquartile range) were recorded. If needed, outcome variables (mean and SD) were estimated from the median and range using formula described by Hozo et al. [17]. Heterogeneity was assessed by I-squared statistics, with > 50% being considered as considerable heterogeneity. A random effects model was applied in cases of considerable heterogeneity (> 50%) and a fixed effects model applied otherwise. Statistical significance was attributed to p values < 0.05.
Systematic review registration
Our systematic review was registered on PROSPERO in December 2021.
Results
Search results
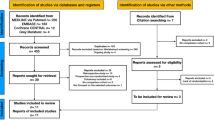
The literature search described above yielded a total of 8214 results (Fig. 1). Following the removal of duplicates, 4804 studies were screened. After the initial screening, 253 abstracts were reviewed and assessed for eligibility. Eighteen were selected for full text review.
From these 18 full texts, 12 were excluded for the following reasons: 5 were an incorrect study design; 3 did not analyse patients undergoing rectal surgery; 3 examined colostomy reversals and 1 did not report on overall morbidity. A total of 6 studies met the inclusion criteria, all of which were included in the quantitative analysis.
Methodological characteristics and quality of studies
All 6 of the included studies were prospective, randomised trials performed in Europe [12, 18,19,20,21,22]. All of the studies were published in English. Table 1 summarises the methodological characteristics of the included studies. The methodological quality of the included studies was generally good and presented in Fig. 2A, B. The GRADE certainty of evidence ranged from low to moderate and is presented in Table 2.
Participant characteristics
The total number of participants in the 6 included studies was 534. Overall, 275 patients underwent EC, with the remaining 259 patients undergoing SC. The baseline characteristics of participants are outlined in Table 3
Overall morbidity
All 6 studies reported overall morbidity rates post-ileostomy closure. Overall morbidity was defined as per the Clavien–Dindo classification. The morbidity rate was 25.5% (70/275) in the EC group and 21.6% (56/259) in the SC group. A meta-analysis of the included studies using the M-H fixed-effects model showed no significant difference between the two groups in regard to overall morbidity rates (OR, 1.24; 95% CI 0.82–1.88; p = 0.31), with moderate heterogeneity reported across the 6 studies (I2 = 44%) (Fig. 3).
Major morbidity (Clavien–Dindo ≥ 3)
All 6 studies reported on major morbidity (Clavien–Dindo > 2) rates post-ileostomy closure. The major morbidity rate was 8% (22/275) in the EC group and 4.6% (12/259) in the SC group. A meta-analysis of the included studies using the M-H fixed-effects model showed no significant difference between the two groups in regard to major morbidity rates (OR, 1.75; 95% CI 0.87–3.53; p = 0.12), with moderate heterogeneity reported across the 6 studies (I2 = 37%) (Fig. 4).
Anastomotic leak (primary anastomosis)
Four studies reported on leak rates of the primary anastomosis after closure of ileostomy [12, 18,19,20]; 5.6% (13/230) in the EC group compared to 4.5% (10/220) in the SC group. A meta-analysis using the M-H fixed-effects model revealed no significant difference in anastomotic leak rates between the two groups (M-H OR, 1.22; 95% CI 0.54–2.76; p = 0.63). Low heterogeneity was found (I2 = 25%) (Fig. 5).
Small bowel obstruction (SBO)/postoperative ileus
All 6 studies reported on SBO/postoperative ileus rates; 4.4% (12/275) in the EC group compared to 9.3% (24/259) in the SC group. A meta-analysis using the M–H fixed-effects model revealed a significant difference in SBO/ileus rates between the two groups, with a significantly lower rate in the EC group (M–H OR, 0.44, 95% CI 0.22–0.90; p = 0.02). No heterogeneity was found (I2 = 0%) (Fig. 6)
Reoperation
All 6 studies reported reoperation rates; 8.4% (23/275) in the EC group compared to 4.2% in the SC group. A meta-analysis using the M–H fixed-effects model revealed no significant difference between the two groups. (M–H OR, 2.02, 95% CI 0.99–4.14; p = 0.06). Moderate heterogeneity was found (I2 = 30%) (Fig. 7)
Postoperative length of stay (LOS)
Three out of the 6 included studies reported on postoperative length of hospital stay [12, 19, 21]. A meta-analysis performed using the random-effects model revealed a marginally longer LOS in the EC group compared to the SC group; however, these results were not significant (MD, 0.44 days longer in the EC group; 95% CI – 0.47 to 1.35; p = 0.34). High heterogeneity was found (I2 = 95%) (Fig. 8).
Operative time
Five studies reported on operative time [12, 18,19,20,21]. A meta-analysis performed using the fixed-effects model revealed a marginally reduced operative time in the EC group; however, these results failed to reach statistical significance (MD, 0.31 min shorter in the EC group; 95% CI – 2.45 to 1.84; p = 0.78). Moderate heterogeneity was found (I2 = 36%) (Fig. 9).
Discussion
We observed no major difference between EC and SC of defunctioning ileostomy in terms rates of overall morbidity, anastomotic leak, length of stay or operative time. While the early closure group did appear to have a higher rate of major morbidity (4.6% vs. 8%), and reoperation (4.2% vs. 8.4%), caution should be taken interpreting these results due to relatively wide 95% confidence intervals and effect estimates. To our knowledge, this is the first meta-analysis to incorporate data from the recently published multicentre RCT performed in Switzerland by Elsner et al [20].
While a defunctioning ileostomy is yet to be shown to reduce the rate of anastomotic leak after rectal surgery, it substantially minimises the sequelae of a leak if one occurs [23]. However, a diverting stoma does not come without its risks, and prolonged exposure can result in a myriad of complications including wound infection, skin excoriation, parastomal hernia, prolapse, dehydration and kidney injury [24]. It is essential to consider not only the physical complications of ileostomy creation, but also the impact it may have on a patient’s quality of life from a psychosocial perspective. Patients often have difficulty adjusting to life with a stoma and may experience anxiety and body image issues relating to their stoma [25]. Where possible, minimising time to reversal may alleviate both the physical complications and psychological impact a stoma may exert on a patient. Unfortunately, to date there is limited data in the literature evaluating the effect of EC vs. SC on patient quality of life.
It is notable that Alves et al. demonstrated the formation of an enterocutaneous fistula in 5 patients in the EC group in comparison to 1 in the SCgroup [18]. In addition, 2 studies were terminated early due to adverse events experienced by patients in their early closure cohorts [12, 20]. Elsner et al. demonstrated significantly higher rates of anastomotic leak (24% vs. 0%, p = 0.002) and reintervention (16% vs. 0%, p = 0.026) in the EC group when compared to SC [20]. Similarly, Bausys et al. reported a higher overall 30-day morbidity rate in the EC group (27.9% vs. 7.9%, p = 0.024) [12]. It is notable that Bausys et al. defined EC as reversal within 30 days, while in all the other studies EC was performed within 8–14 days of primary rectal resection, which may have impacted the outcomes. While it cannot be stated with any certainty, it is possible that these studies may have had a more significant impact on the outcome in favour of SC had they been allowed to continue. Conversely, the EASY study found a considerably lower mean number of complications in their EC group when compared to SC [19]. Unlike other included studies, Danielsen et al. carefully selected participants deemed fit to participate in the study, who showed no signs of infections or leakage. 127 participants out of a total of 418 were deemed eligible to participate. This study demonstrated the feasibility and safety of EC in “well-selected” patients, showing no clinical, radiological or endoscopic signs of leakage. While it may be premature to say with certainty that EC is safe without further studies, there is certainly evidence to suggest its feasibility in certain cohorts. Further research is required to help define the characteristics of this ‘optimal’ cohort of patients.
The RCTs of Elsner and Alves et al. were the only 2 to examine the impact of EC on patient quality of life. The authors observed an improved quality of life at 6 weeks and 4 months, and 3 months and 12 months, respectively, in patients who underwent EC, but no longer term data is available [18, 20]. In contrast, Elsner et al.found comparable Gastrointestinal Quality of Life Index (GQLI) scores between the two groups at both 6 weeks and 4 months after low anterior resection (EC 106/144, SC 109/144). This data was supported by the use of a second quality of life questionnaire (EORTC-QLQ-C30) which yielded similar results [20]. Alves et al. had similar results in their study, with a GQLI score of 111 and 108 in the EC and SC groups, respectively [18]. Considering two RCTs were halted prematurely for safety concerns, it may be more realistic to analyse long-term data on functional outcomes and quality of life via a cohort study. If these outcomes proved to be superior in the EC group, the benefits of EC may outweigh the risks, when presented to patients.
It is evident that we need more studies comparing quality of life outcomes between patients undergoing EC and SC of ileostomy after rectal surgery. Improved subgroup analysis particularly looking at quality of life outcomes in terms of patient age, functional outcomes, development of LARS and acute kidney injury (AKI) rates may provide evidence that will guide surgeons in their careful consideration and selection of patients for early stoma closure. Unfortunately, we were unable to fully assess the functional components of the closure groups in our review, as several of the studies failed to utilise a validated scoring tools and did not allow for adequate follow-up time. Patient-reported outcome measures (PROMs) allow surgeons to gain insight from the perspective of the patient into how surgery impacts their lifestyle and quality of life [26, 27]. Standardised questionnaires that to collect data on patients postoperatively, particularly regarding symptoms, health-related quality of life and functional status are vital [28]. Despite an increasing amount of support for the use of PROMs in the literature, there has been limited uptake amongst surgeons regarding this topic [29, 30]. A large scale RCT investigating PROMs in these two cohorts of patients would provide invaluable data to assist us in our selection of patients for early closure. Furthermore, more data is required on outcomes of early closure in patients undergoing adjuvant chemotherapy. While several small observational studies have demonstrated the feasibility and safety of early closure in patients undergoing adjuvant therapy, a large RCT is necessary to compare outcomes between these two groups [31,32,33]. Timing of the closure should be considered on a case by case basis with careful patient selection and good perioperative counselling of all potential risks highlighted by this review. If opting for early reversal, a preoperative gastrograffin enema or computed tomography scan is essential to exclude an anastomotic leak prior to reversal [34, 35].
An alternative to routine diversion, known as the’selective diversion’ approach, has been postulated and demonstrated to be feasible, albeit with limited evidence [36]. This approach aims to avoid stoma-related morbidity by offering proactive leak management only when a leak is suspected [37]. While there is limited evidence to suggest its safety, the authors believe the risks of this approach do not outweigh the benefits. Anastomotic leak and subsequent pelvic sepsis has been shown to significantly increase the risk of developing LARS [38]. The formation of a loop ileostomy to divert bowel contents away from the site of anastomosis is a well-established risk management strategy known to minimise the sequelae of an anastomotic leak and international widespread change is unlikely [6].
This review has several strengths and provides evidence in terms of outcome data that would help inform surgeon–patient consultation. Data was only included from randomised controlled trials (534 patients) and had low levels of heterogeneity. It provides clear outcome data to help guide decision-making and counsel patients preoperatively in terms of recovery, expected morbidity and/or re-operation. It could, for example, be a safer option in elderly patients, who are at increased risk of dehydration and acute kidney injury secondary to a prolonged ileostomy losses [39]. We do acknowledge that the review has some limitations, particularly in terms of impact of age, comorbidities and extent of primary surgery on the selection criteria for early closure. Despite this, the authors are of the opinion that EC can be considered in highly selective cases with adequate perioperative counselling. In addition, there remains a lack of data on quality of life outcomes across the majority of included studies. Another limitation is the inability to divide the SBO and postoperative ileus data into separate groups to provide a more accurate analysis and draw more realistic conclusions on the rate of obstruction in the two groups. Despite these limitations, this meta-analysis highlights the feasibility of early stoma closure in a select cohort of patients who are well counselled on both the risks and benefits of this method of reversal.
Conclusions
For most outcomes, out review observed no difference between EC and SC of defunctioning ileostomy after rectal surgery. However, the EC group did have a higher rate of major morbidity and/or reoperation. Overall, early closure appears to be a feasible option in highly selective cases with good perioperative counselling and shared decision-making. The impact of EC on overall quality of life requires further evaluation.
References
Anderin K et al (2016) The effect of diverting stoma on long-term morbidity and risk for permanent stoma after low anterior resection for rectal cancer. Eur J Surg Oncol 42(6):788–793
Fukudome I et al (2021) The safety of early versus late ileostomy reversal after low anterior rectal resection: a retrospective study in 47 patients. Patient Saf Surg 15(1):7
Jafari MD (2013) Morbidity of diverting ileostomy for rectal cancer: analysis of the American College of Surgeons National Surgical Quality Improvement Program. Am Surg 79(10):1034–1039
Dukes’ Club Research, C (2021) Factors impacting time to ileostomy closure after anterior resection: the UK closure of ileostomy timing cohort study (CLOSE-IT). Colorectal Dis 23(5):1109–1119
Omundsen M et al (2012) Early ileostomy closure: is there a downside? ANZ J Surg 82(5):352–354
Dulskas A et al (2021) Quality of life and bowel function following early closure of a temporary ileostomy in patients with rectal cancer: a report from a single-center randomized controlled trial. J Clin Med 10(4):768
Audit NBC (2021) Annual Report 2021
Uimonen M et al (2021) The impact of the COVID-19 pandemic on waiting times for elective surgery patients: a multicenter study. PLoS ONE 16(7):e0253875
Massucco P et al (2021) Prospective, randomised, multicentre, open-label trial, designed to evaluate the best timing of closure of the temporary ileostomy (early versus late) in patients who underwent rectal cancer resection and with indication for adjuvant chemotherapy: the STOMAD (STOMa closure before or after ADjuvant therapy) randomised controlled trial. BMJ Open 11(2):e044692
Keane C et al (2019) Functional outcomes from a randomized trial of early closure of temporary ileostomy after rectal excision for cancer. Br J Surg 106(5):645–652
Baek SJ et al (2014) Relationship between the severity of diversion colitis and the composition of colonic bacteria: a prospective study. Gut Liver 8(2):170–176
Bausys A et al (2019) Early versus standard closure of temporary ileostomy in patients with rectal cancer: a randomized controlled trial. J Surg Oncol 120(2):294–299
Vogel I et al (2021) Impact of a defunctioning ileostomy and time to stoma closure on bowel function after low anterior resection for rectal cancer: a systematic review and meta-analysis. Tech Coloproctol 25(7):751–760
Aljorfi AA, Alkhamis AH (2020) A systematic review of early versus late closure of loop ileostomy. Surg Res Pract 2020:9876527
Higgins JPT et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Guyatt GH et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Alves A et al (2008) Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy. Br J Surg 95(6):693–698
Danielsen AK et al (2017) Early closure of a temporary ileostomy in patients with rectal cancer: a multicenter randomized controlled trial. Ann Surg 265(2):284–290
Elsner AT et al (2021) Closure of temporary ileostomy 2 versus 12 weeks after rectal resection for cancer: a word of caution from a prospective, randomized controlled multicenter trial. Dis Colon Rectum 64(11):1398–1406
Klek S et al (2018) Early closure of the protective ileostomy after rectal resection should become part of the Enhanced Recovery After Surgery (ERAS) protocol: a randomized, prospective, two-center clinical trial. Wideochir Inne Tech Maloinwazyjne 13(4):435–441
Lasithiotakis K, Aghahoseini A, Alexander D (2016) Is early reversal of defunctioning ileostomy a shorter, easier and less expensive operation? World J Surg 40(7):1737–1740
Wong NY, Eu KW (2005) A defunctioning ileostomy does not prevent clinical anastomotic leak after a low anterior resection: a prospective, comparative study. Dis Colon Rectum 48(11):2076–2079
Mehboob A et al (2020) Frequency and complications of ileostomy. Cureus 12(10):e11249
Nugent KP et al (1999) Quality of life in stoma patients. Dis Colon Rectum 42(12):1569–1574
Meadows KA (2011) Patient-reported outcome measures: an overview. Br J Community Nurs 16(3):146–151
Mason SJ et al (2018) Evaluating patient-reported outcome measures (PROMs) for bladder cancer: a systematic review using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist. BJU Int 122(5):760–773
Churruca K et al (2021) Patient-reported outcome measures (PROMs): A review of generic and condition-specific measures and a discussion of trends and issues. Health Expect 24(4):1015–1024
Mou D et al (2020) The surgeon’s Perceived Value of Patient-reported Outcome Measures (PROMs): an exploratory qualitative study of 5 different surgical subspecialties. Ann Surg 275:500–505
Sokas C et al (2022) A review of PROM implementation in surgical practice. Ann Surg 275(1):85–90
Zhen L et al (2017) Effectiveness between early and late temporary ileostomy closure in patients with rectal cancer: a prospective study. Curr Probl Cancer 41(3):231–240
Tulchinsky H et al (2014) Should a loop ileostomy closure in rectal cancer patients be done during or after adjuvant chemotherapy? J Surg Oncol 109(3):266–269
Gu X (2020) Early ileostomy closure is safe and feasible during adjuvant chemotherapy after total mesorectal excision surgery for rectal cancer. J Clin Oncol 38:4111
Khair G et al (2007) Routine use of gastrograffin enema prior to the reversal of a loop ileostomy. Dig Surg 24(5):338–341
Sherman KL, Wexner SD (2017) Considerations in stoma reversal. Clin Colon Rectal Surg 30(3):172–177
Talboom K et al (2021) Highly selective diversion with proactive leakage management after low anterior resection for rectal cancer. Br J Surg 108(6):609–612
Blok RD et al (2018) Impact of an institutional change from routine to highly selective diversion of a low anastomosis after TME for rectal cancer. Eur J Surg Oncol 44(8):1220–1225
Kim S et al (2021) The effect of anastomotic leakage on the incidence and severity of low anterior resection syndrome in patients undergoing proctectomy: a propensity score matching analysis. Ann Coloproctol 37(5):281–290
Smith SA et al (2021) New ileostomy formation and subsequent community-onset acute and chronic kidney disease: a population-based cohort study. Ann Surg 274(2):352–358
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors report no conflict of interest.
Ethical and Informed Consent statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Sullivan, N.J., Temperley, H.C., Nugent, T.S. et al. Early vs. standard reversal ileostomy: a systematic review and meta-analysis. Tech Coloproctol 26, 851–862 (2022). https://doi.org/10.1007/s10151-022-02629-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-022-02629-6