Abstract
The purpose of this study was to assess outcome measures and cost-effectiveness of robotic colorectal resections in adult patients with inflammatory bowel disease. The Cochrane Library, PubMed/Medline and Embase databases were reviewed, using the text “robotic(s)” AND (“inflammatory bowel disease” OR “Crohn’s” OR “Ulcerative Colitis”). Two investigators screened abstracts for eligibility. All English language full-text articles were reviewed for specified outcomes. Data were presented in a summarised and aggregate form, since the lack of higher-level evidence studies precluded meta-analysis. Primary outcomes included mortality and postoperative complications. Secondary outcomes included readmission rate, length of stay, conversion rate, procedure time, estimated blood loss and functional outcome. The tertiary outcome was cost-effectiveness. Eight studies (3 case-matched observational studies, 4 case series and 1 case report) met the inclusion criteria. There was no reported mortality. Overall, complications occurred in 81 patients (54%) including 30 (20%) Clavien-Dindo III–IV complications. Mean length of stay was 8.6 days. Eleven cases (7.3%) were converted to open. The mean robotic operating time was 99 min out of a mean total operating time of 298.6 min. Thirty-two patients (24.7%) were readmitted. Functional outcomes were comparable among robotic, laparoscopic and open approaches. Case-matched observational studies comparing robotic to laparoscopic surgery revealed a significantly longer procedure time; however, conversion, complication, length of stay and readmission rates were similar. The case-matched observational study comparing robotic to open surgery also revealed a longer procedure time and a higher readmission rate; postoperative complication rates and length of stay were similar. No studies compared cost-effectiveness between robotic and traditional approaches. Although robotic resections for inflammatory bowel disease are technically feasible, outcomes must be interpreted with caution due to low-quality studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic colorectal resections are now routinely performed worldwide for patients with inflammatory bowel disease (IBD). Laparoscopy may be associated with less pain, reduced intra-abdominal adhesions, shorter duration of hospitalisation and quicker return to function compared to open surgery [1,2,3]. However, potential disadvantages include amplification of hand tremors and loss of wrist movement due to limited movement of the long instruments within the trocars [4]. Consequently, robotic platforms, such as the da Vinci® system, have been developed in order to address these limitations. The theoretical advantages of these systems include a stable camera platform, three-dimensional image, excellent ergonomic function with ambidextrous capability and freedom of movement in multiple directions [5].
The first robotic colorectal procedure was performed in New Jersey, USA, in 2001 [6], and this approach has gained considerable support over the past 15 years, especially for pelvic surgery. Early studies demonstrated that robotic total mesorectal excision (TME) for rectal cancer was safe and feasible and achieved a low number of positive resection margins and low conversion rates [7, 8]. Indeed, early results from the first international, multi-centre randomised controlled trial of 471 patients comparing robotic with laparoscopic TME revealed similar oncological clearance, patient outcomes and conversion to open surgery rates [9]. Additionally, it has been proposed that it may reduce the risk of complications related to pelvic nerve injuries [10]. However, the advantages with respect to abdominal resections is less clear, as a randomised controlled study did not report any benefits associated with the use of robotic assistance compared with laparoscopic right hemicolectomy [11]. Furthermore, the expense of installing and maintaining these platforms is significant and potentially prohibits their widespread use, particularly in an environment of limited health care resources. For instance, a recent American study comparing robotic to laparoscopic colectomies found a mean cost increase of approximately $15,595 per case [12].
Evidence to justify the use of robotic colorectal resection in patients with IBD remains even sparser. Therefore, the aim of this study was to perform a systematic review of the published literature in order to report the clinical outcomes and cost associated with robotic colorectal resections for patients with IBD.
Materials and methods
Search strategy
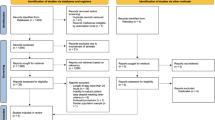
PubMed (1966–September 2016), Medline (1946–September 2016), the Cochrane Library and Embase (1947–September 2016) were electronically searched using the following text: “robotic(s)” AND (“inflammatory bowel disease” OR “Crohn’s” OR “ulcerative colitis”). In addition, reference lists of relevant articles, reviews and commentaries were manually searched, and experts in the specialty were contacted to identify papers not captured by electronic searches (Fig. 1). Studies searched were limited to those performed in adult humans and published in the English language. Furthermore, if the abstract or full manuscript was irrelevant or contained insufficient data (such as absence of subgroup analysis), it was excluded from the analysis.
Study quality assessment
Quality assessment was performed using the National Institute for Health and Care Excellence (NICE), Quality Assessment for Case Series (QACS) tool [13] by scoring the studies out of a maximum of 8 points. A study scored 1 point each if; (a) it was multi-centre, (b) hypothesis/aim/objective clearly reported, (c) outcomes defined, (d) inclusion and exclusion criteria stated, (e) data prospectively collected, (f) patients consecutively recruited and (g) the main findings of the study are clearly described and (h) outcomes stratified.
Study outcomes
The primary outcome measures of interest included mortality and postoperative complications, classified according to Clavien-Dindo grade I–IV [14]. Secondary outcome measures included: (i) readmission rates (ii) length of postoperative stay (LOS), (iii) conversion to open surgery rate, (iv) mean operating time, (v) estimated blood loss, (vi) functional outcomes. The tertiary outcome measure was cost-effectiveness, by comparing the cost of robotic procedures to its laparoscopic equivalent.
Data extraction
Quantitative data were extracted by 2 independent reviewers (SR/AH), and results were cross-checked. Any discrepancies in results were resolved by repeat data extraction, discussion and further review of the index study.
Data analysis
Given that the majority of studies lacked a control group, meta-analysis of the data was precluded. As such, the results from each study are presented in a summarised and aggregate form.
Results
Search results
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [15] were adhered to for the reporting of this systematic review. The electronic search yielded a total of 60 citations. Following examination of the full-text manuscript (n = 43), a final total of 8 studies were eligible for inclusion (Fig. 1). They included 150 patients; 10 with a diagnosis of Crohn’s disease, 2 with indeterminate colitis and 138 with ulcerative colitis (UC).
Study characteristics and quality assessment
One of the 8 studies was a case report, 4 were case studies, and 3 were retrospective case-matched reviews comparing robotic to laparoscopic surgery in 2 studies and to open surgery in 1 study [16,17,18,19,20,21,22,23]. Quality assessment is recorded in Table 1.
Type of procedure performed
The type of procedure performed in each of the studies varied as well as the extent of robotic involvement (Table 1). The most extensive involvement of the robotic system was used by Roviello et al. [23] in 4 patients involving a single-docking robotic proctocolectomy with formation of a terminal ileostomy. A conventional laparoscopic port was also used during these procedures, principally to assist with additional traction. The remaining studies performed robotic proctectomy with or without variable amounts of laparoscopic assistance ± laparoscopic colectomy ± extracorporeal formation of a J-pouch with formation of a hand-sewn/stapled (open or laparoscopic) ileal pouch-anal anastomosis (IPAA) via a perineal/Pfannenstiel/suprapubic incision ± diverting loop ileostomy (Table 1). Further classification regarding the exact type of procedure for each patient was not recorded in every study [16].
Primary outcomes
Mortality
There was no mortality in any of the studies.
Postoperative complications
Complications occurred in 81 patients (54%) including serious complications (Clavien-Dindo grade III–IV) in 30 patients (20%). Early postoperative morbidity varied from 0 to 100% (Table 2). 100% of the high-risk patients operated on with fulminant UC by McLemore et al. [18] experienced early complications, although all complications were a maximum of Clavien-Dindo II. The single case report did not describe any complications [20]. The remaining studies reported a morbidity rate of 40–75%. Rencuzogullari et al. [22] reported no statistically significant difference between postoperative complications following robotic and laparoscopic approaches, with similar postoperative outcomes reported in the second case-matched study (although no p value was obtained) [19]. The single case-matched retrospective study comparing robotic to open proctectomy also reported no statistically significant differences in postoperative complications between the two groups [17]. In this study, 10% of the patients undergoing robotic surgery underwent a reoperation, which was statistically similar to the 5% in the control group (p = 0.18).
Secondary outcomes
Readmission rates
Overall, 37 patients (24.7%) were readmitted. Mark-Christensen et al. [17] reported a readmission rate of 40%, which is a significantly higher rate than that seen in the open surgery group (p = 0.02 on univariate analysis and p = 0.03 on multivariate analysis). Rencuzogullari et al. [22] reported no significant difference in readmission rate between the robotic and laparoscopic group (p = 1.0).
Length of stay (LOS)
Overall, the mean LOS for 146 patients was 8.6 days, ranging from 5.3 ± 1.2 to 15 days (Table 1). The 4 patients in the study by Roviello et al. [23] were excluded as only a median LOS was reported. Mark-Christensen et al. [17] reported a mean LOS of 9.1 ± 5 days following robotic surgery which was significantly shorter than the 11 ± 6.4 days following open surgery (p = 0.02), however in multivariate regression analysis taking into account the primary operation, body mass index, American Society of Anaesthesiologists classification, sex and age this finding was no longer significant (p = 0.07). Rencuzogullari et al. [22] reported a mean LOS of 7.9 ± 6.4 days which was not statistically significant from their laparoscopic group (p = 0.39). Miller et al. [19] also reported no significant difference in mean LOS between colectomised patients who underwent robotic proctectomy (RP) + IPAA (RP–IPAA) (8.5 ± 3.8 days) and those who underwent laparoscopic proctectomy (LP) + IPAA (LP–IPAA) (6.1 ± 2.2 days; p = 0.17); however, there was a significantly longer mean LOS for robotic completion proctectomy (RP-CP) (6.4 ± 1.0 days) compared with laparoscopic completion proctectomy (LP-CP) (4.1 ± 0.7 days; p = 0.02).
Conversion rates and estimated blood loss (EBL)
Overall, 11 robotic procedures were converted to open (7.3%). Six studies reported a conversion rate of zero, including analysis of 48 patients. The remaining 2 studies [17, 22] reported a conversion rate of 11.1 and 9.5%, respectively. Reasons for conversion in reported by Rencuzogullari et al. [22] included 1 case of extensive adhesions and 1 case of unclear anatomy. There was no significant difference in conversion rate between robotic and laparoscopic approaches [22].
EBL was recorded in 6 studies (Table 1), involving 66 patients, with a wide variation: 57.5–486 ml [19, 20]. Overall, mean (median of the means) EBL was 248.5 ml. Of note, Miller et al. [19] did not show a significant difference in EBL between RP-CP and LP-CP (p = 0.18), or between RP–IPAA and LP–IPAA (p = 0.15). However, Rencuzogullari et al. [22] reported a significantly higher EBL for the robotic compared to laparoscopic approach (p = 0.002).
Procedure time
Direct comparison of the reported procedure time was difficult due to the heterogeneity of procedures between the studies (Table 1). Additionally, some studies only reported total operative time rather than a robotic time. However, the overall mean robotic operating time (median of the means) was 99 min out of a mean total operative time of 298.6 min.
McLemore et al. [18] reported a mean ± SD robotic time of 123 ± 14.9 min out of a total procedure time of 436 ± 106.6 min. This total procedure time is notable, especially considering that all patients had previously undergone laparoscopic colectomy. However, this cohort underwent surgery for acute, severe disease with a diagnosis of fulminant UC. Roviello et al. [23] reported the shortest total procedure time 235 min and interestingly performed the most robotic surgery involving robotic colectomy in addition to proctectomy, although no anastomosis was performed.
Miller et al. [19] directly compared total procedure time ± SD for colectomised patients undergoing RP-CP (351 ± 76.3 min) to LP-CP (238 ± 66.4 min), and there was a significant time increase with robotic procedures (p = 0.03). However, this difference was not significant for colectomised patients undergoing RP–IPAA (370 ± 65.9 min) compared to LP–IPAA (316 ± 78.4 min; p = 0.14). Rencuzogullari et al. [22] reported a mean total operative time ± SD of 304 ± 109 min for robotic proctectomy ± laparoscopic colectomy ± IPAA which was significantly longer than the equivalent laparoscopic procedure (213 ± 86 min; p = 0.008). Finally, as would be expected, Mark-Christensen et al. [17] reported a significantly longer total operative time ± SD for robotic proctectomy ± laparoscopic colectomy + IPAA (284 ± 38 min) compared to the equivalent open procedure (130 ± 38 min), which was significant in both univariate and multivariate analysis (p = <0.01).
Functional outcomes
The 3 case-matched studies reported similar long-term pouch outcomes between robotic and laparoscopic or open procedures. Mark-Christensen et al. [17] reported 1 episode of pouch failure in the robotic group (p = 0.97), whilst Rencuzogullari et al. [22] reported 1 episode of pouch fistula (p > 0.99) and 1 episode of anastomotic separation (p > 0.99). Miller et al. [19] reported similar functional outcomes between RP–IPAA and LP–IPAA after reversal of ileostomy. Although the numbers are small, there was no significant difference in pouch function and continence, specifically anal continence during daytime and night time (p = 0.58), minor leakage (p = 0.58), frequency of bowel movements (p = 0.15), ability to postpone bowel movements (p = 0.30) and anal pruritus (p = 0.14) between these cohorts. Post-procedure quality of life scores (p = 1.0), as well as sexual functional outcome measures including change in sexual desire (p = 0.66) and quality of erection (p = 1.0), were equivalent in the 2 groups [19].
Mean return of bowel function in days, following robotic proctectomy, was reported in 3 studies. Rencuzogullari et al. [22] reported a mean ± SD of 2.3 ± 1.5 days equivalent to that seen in its laparoscopic group (p = 0.62). This length was comparable to mean return of bowel function reported by Miller et al. [19] for RP-CP (3.0 ± 0.8 days) and RP–IPAA (3.6 ± 2.8 days). Although return of bowel function following RP-CP took significantly longer than after LP-CP (p = 0.04), it was statistically equivalent for RP–IPAA and LP–IPAA (p = 0.3). Pedraza et al. [21] reported a mean ± SD of 2.4 ± 0.9 days to return of bowel function following robotic proctectomy + laparoscopic colectomy + IPAA, which was broadly equivalent to the other 2 studies. Mean number of days to normal diet was reported in 2 studies. Pedraza et al. [21] reported a mean of 2 ± 0.6 days which was comparable to that reported by Byrn et al. (4.7 ± 2.9 and 3.0 ± 0.6) [16].
Tertiary outcomes
Cost implications
Only 1 study reported a cost analysis [16]. Direct costs observed for robotic IBD cases, excluding cost of acquisition, depreciation, amortisation and maintenance of the robotic platform, showed a decreasing trend over a period of 27 months ($19,278 ± 13,404 vs. $13,413 ± 2504; p = 0.06). The ratio of observed to expected cost, which aims to correct for patient-specific factors that increase cost outside of the surgical procedure, decreased over time (1.8 ± 0.8 vs. 1.2 ± 0.1; p = 0.02) perhaps as a result of decreased operating time and length of stay. No study compared the cost of robotic surgery to traditional approaches.
Discussion
This systematic review identified 8 studies reporting outcomes following robotic colorectal resection in 150 patients with IBD. Notably, there were no randomised trials; however, 2 retrospective studies showed comparable results between robotic and laparoscopic surgery and 1 between robotic and open surgery. The studies were heterogeneous in terms of the populations studied, procedures performed and criteria by which outcomes were measured, precluding formal meta-analysis.
Primary outcomes
The rate of reported pelvic sepsis, arguably the most significant early complication following IPAA, in each of the studies analysed in this review fell within the range reported following IPAA in a meta-analysis of 43 observational studies (range 2.3–26.7%) [24]. However, the overall morbidity rate of 54% is higher than recent published literature evaluating laparoscopic surgery for IBD (Table 3) [25,26,27,28,29,30,31,32,33,34]. Although most studies defined the morbidity rate as the number of patients affected by complications [18, 20, 21, 23, 25,26,27,28,29, 31, 32, 34], several studies, particularly those in this review [16, 19, 22], did not specify how many patients were affected.
There was no significant difference in early postoperative complications between robotic and open surgery following IPPA [17], largely consistent with early meta-analyses of laparoscopic versus open studies during the ascending phase of the laparoscopic learning curve which reported, aside from a lower incidence of wound infection in laparoscopic surgery [35], equivalent adverse event rates between the 2 groups [35,36,37]. More recent studies indicate that laparoscopic surgery is associated with fewer early complications and lower rates of pelvic sepsis [39, 40] than open surgery [28, 33, 38]. However, the supplementary use of mini-laparotomies or Pfannenstiel incisions in robotic surgery is often necessary and may limit the benefits that have been seen in other minimally invasive surgical procedures. In the study by Mark-Christensen et al. [17], rectal stapling was performed using non-endoscopic staplers in one-third of the robotic cases, to ensure an adequate level and completeness of the stapling, emphasising an important technical challenge to this approach [17].
The case-matched robotic and laparoscopic studies [19, 22] also showed comparable morbidity rates, although 1 study did not analyse p values [19]. Studies evaluating the adoption of robotic technology for rectal cancer patients suggest 3 phases, with the first phase of learning achieved within a range of 9–41 cases [41,42,43]. It is likely, therefore, that the surgeons in these case-matched studies were in their initial learning curve of robotic surgery, though masters in laparoscopy and yet, robotic surgery provided comparable outcomes [17, 22, 29]. The absence of randomised controlled trials, however, makes any definitive conclusions difficult. Furthermore, the ROLARR trial has not shown any reduction in 30-day morbidity in robotic compared to laparoscopic rectal cancer resections [9].
Secondary outcomes
Overall secondary outcomes were consistent with published laparoscopic studies (Table 3) [25,26,27,28,29,30,31,32,33,34].
Robotic readmission rates were similar to rates reported in laparoscopic case-matched controls [19, 22] although higher than in open surgery [17]. Importantly, this was not reflected in a higher major complication rate on readmission, indicating that there may be a lower threshold to readmit trial patients undergoing minimally invasive surgery [17]. Of note, the period during which readmission was analysed was not always declared and as such may represent a source of bias.
LOS was statistically similar to case-matched laparoscopic and open studies [17, 22]. Miller et al. [19] proceeded with RP-CP, in order to establish and optimise robotic surgical technique and to avoid affecting long-term functional outcomes associated with restorative procedures, which may go some way to explain the better results they achieved with RP–IPAA as they had accumulated more experience. This is supported by Byrn et al. [16] who reported a significant reduction in mean LOS following robotic proctectomy over a 2-year period (p = 0.03), suggesting that recovery after robotic surgery may be quicker when performed by experienced operators. Of note, temporal and spatial patterns in adherence to principles of fast-track surgery may represent a substantial source of bias; the paper with the largest number of patients collected data over a 10-year period, from 2004 to 2014 [17].
Although there was no significant difference in conversion rates between case-matched robotic and laparoscopic procedures [22], it is important to note that conversion rate is a subjective endpoint. Overall EBL was consistent with EBL in laparoscopic studies (Table 3).
Procedure time was significantly longer than the time reported for case-matched laparoscopic [22] and open procedures [17]; however, the overall operative time appears comparable to published laparoscopic literature (Table 3). Conceivably, the operative time may decrease with increasing experience as was seen in the study by Miller et al. [19] where later procedures (RP–IPAA) were not significantly longer than their laparoscopic equivalent. Additionally, Byrn et al. [16] and Mark-Christensen et al. [17] showed a trend towards a decrease in total procedure time for robotic procedures over the course of their studies, although it was not clear at what stage of the procedure the time was shortened and the improvement was not statistically significant. Newer platforms, or hybrid procedures involving laparoscopic with robotic techniques, may help to reduce the necessity of multiple docking and operative times [22].
The ultimate purpose of IPAA surgery is to ensure satisfactory pouch function, which is an outcome that many papers did not assess. Heterogeneity of functional outcome measured makes comparison difficult; however, the case-matched studies showed no difference in days to return of bowel function following robotic or laparoscopic IPAA [19, 22] or pouch failure rates [17, 22]. One study reported no differences in pouch function, quality of life and sexual function after robotic or laparoscopic procedures [19]. It is worthwhile noting that the majority of patients with IBD are young and therefore future studies should include functional, including sexual, data analysis.
Tertiary outcomes
The overall economic feasibility of robotic surgery for IBD was not determined, as only 1 study, without a control group, assessed the cost of robotic surgery [16]. Currently, it seems likely that high costs will prevent widespread adoption of robotic surgery in the near future, particularly without any evidence of improved outcomes.
Limitations
In addition to those limitations already discussed, the most important limitation of this review is the low-quality papers analysed, mainly observational, single-centre, single-surgeon, retrospective, non-randomised designs with low patient numbers. Additionally, the analysed papers did not have subgroup analyses, which made comparing results for one specific operation or a specific disease (e.g. Crohn’s or UC) impossible. As such, patients with different diseases, preoperative conditions and operations were compared, leading to bias when comparing postoperative outcome. Finally, analysing articles in the English language only has limited the coverage of the review.
Conclusions
Outcome data of robotic surgery for IBD must be interpreted with caution due to low-quality studies. However, robotic resections in patients with IBD are technically feasible. The significantly higher overall costs necessitate evidence for advantages over traditional approaches. Thus far, no such advantages have been demonstrated precluding a recommendation for widespread adoption.
References
Wu XJ, He XS, Zhou XY, Ke J, Lan P (2010) The role of laparoscopic surgery for ulcerative colitis: systematic review with meta-analysis. Int J Colorectal Dis 25(8):949–957
Telem DA, Vine AJ, Swain G et al (2010) Laparoscopic subtotal colectomy for medically refractory ulcerative colitis: the time has come. Surg Endosc 24(7):1616–1620
Indar AA, Efron JE, Young-Fadok TM (2009) Laparoscopic ileal pouch-anal anastomosis reduces abdominal and pelvic adhesions. Surg Endosc 23(1):174–177
Wexner SD, Bergamaschi R, Lacy A et al (2009) The current status of robotic pelvic surgery: results of a multinational interdisciplinary consensus conference. Surg Endosc 23(2):438–443
Tou S, Pavesi E, Nasser A, Mazirka P, Bergamaschi R (2015) Robotic-assisted strictureplasty for Crohn’s disease. Tech Coloproctol 19(4):253–254
Weber PA, Merola S, Wasielewski A, Ballantyne GH (2002) Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 45(12):1689–1694 (discussion 95-6)
Baik SH, Kwon HY, Kim JS et al (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16:1480–1487
Pigazzi A, Luca F, Patriti A et al (2010) Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol 17:1614–1620
American Society of Colon and Rectal Surgeons (ASCRS) (2015). www.fascrs.org/video/results-robotic-vs-laparoscopic-resection-rectal-cancer-rolarr-study-2015. Accessed 15 Sept 2016
Pigazzi A, Garcia-Aguilar J (2010) Robotic colorectal surgery: for whom and for what? Dis Colon Rectum 53(7):969–970
Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP (2012) Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 99:1219–1226
Moghadamyeghaneh Z, Hanna MH, Carmichael JC et al (2016) Comparison of open, laparoscopic and robotic approaches for total abdominal colectomy. Surg Endosc 30:2792–2798
National Institute for Health and Care Excellence (2016). https://www.nice.org.uk/guidance/cg3/resources/appendix-4-quality-of-case-series-form2. Accessed Sept 2016
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med 6(7):e1000100
Byrn JC, Hrabe JE, Charlton ME (2014) An initial experience with 85 consecutive robotic-assisted rectal dissections: improved operating times and lower costs with experience. Surg Endosc 28(11):3101–3107
Mark-Christensen A, Pachley FR, Norager CB, Jepsen P, Laurberg S, Tottrup A (2016) Short-term outcome of robot-assisted and open IPAA: an observational single-center study. Dis Colon Rectum 59:201–207
McLemore EC, Cullen J, Horgan S, Talamini MA, Ramamoorthy S (2012) Robotic-assisted laparoscopic stage II restorative proctectomy for toxic ulcerative colitis. Int J Medic Robot Comput Assist Surg MRCAS 8(2):178–183
Miller AT, Berian JR, Rubin M, Hurst RD, Fichera A, Umanskiy K (2012) Robotic-assisted proctectomy for inflammatory bowel disease: a case-matched comparison of laparoscopic and robotic technique. J Gastrointest Surg 16(3):587–594
Morelli L, Guadagni S, Mariniello MD et al (2015) Hand-assisted hybrid laparoscopic-robotic total proctocolectomy with ileal pouch–anal anastomosis. Langenbecks Arch Surg 400(6):741–748
Pedraza R, Patel CB, Ramos-Valadez DI, Haas EM (2011) Robotic-assisted laparoscopic surgery for restorative proctocolectomy with ileal J pouch-anal anastomosis. Minim Invasive Ther Allied Technol 20(4):234–239
Rencuzogullari A, Gorgun R, Costedio M et al (2016) Case-matched comparison of robotic versus laparoscopic proctectomy for inflammatory bowel disease. Surg Laparosc Endosc Percutan Tech 26(3):37–40
Roviello F, Piagnerelli R, Ferrara F, Scheiterle M, De Franco L, Marrelli D (2015) Robotic single docking total colectomy for ulcerative colitis: first experience with a novel technique. Int J Surg 21:63–67
Hueting WE, Buskens E, van der Tweel I et al (2005) Results and complications after ileal pouch anal anastomosis: a meta-analysis of 43 observational studies comprising 9317 patients. Dig Surg 22:69–79
El-Gazzaz GS, Kiran RP, Remzi FH et al (2009) Outcomes for case-matched laparoscopically assisted versus open restorative proctocolectomy. Br J Surg 96:522–526
Lefevre JH, Bretagnol F, Ouaissi M et al (2009) Total laparoscopic ileal pouch-anal anastomosis: prospective series of 82 patients. Surg Endosc 23(1):166–173
Fichera A, Silvestri MT, Hurst RD et al (2009) Laparoscopic restorative proctocolectomy with ileal pouch anal anastomosis: a comparative observational study on long-term functional results. J Gastrointest Surg 13(3):526–532
Fajardo AD, Dharmarajan S, George V et al (2010) Laparoscopic versus open 2-stage ileal pouch: laparoscopic approach allows for faster restoration of intestinal continuity. J Am Coll Surg 211:377–383
Fleming FJ, Francone TD, Kim MJ et al (2011) A laparoscopic approach does reduce short-term complications in patients undergoing ileal pouch-anal anastomosis. Dis Colon Rectum 54:176–182
Dolejs S, Kennedy G, Heise C (2011) Restorative Proctocolectomy: affected by a laparoscopic approach? J Surg Res 170(2):202–208
Goede AC, Reeves A, Dixon AR (2011) Laparoscopic restorative proctocolectomy a 10-year experience of an evolving technique. Colorectal Dis 13(10):1153–1157
Duff SE, Sagar PM, Rao M et al (2012) Laparoscopic restorative proctocolectomy: safety and critical level of the ileal pouch anal anastomosis. Colorectal Dis 14(7):883–886
Schiessling S, Leowardi C, Kienle P et al (2013) Laparoscopic versus conventional ileoanal pouch procedure in patients undergoing elective restorative proctocolectomy (LapConPouchTrial)—a randomized controlled trial. Langenbecks Arch Surg 398:807–816
Inada R, Nagasaka T, Kondo Y et al (2015) A case-matched comparative study of laparoscopic and open total proctocolectomy for ulcerative colitis. Acta Med Okayama 69:267–273
Singh P, Bhangu A, Nicholls RJ, Tekkis P (2013) A systematic review and meta-analysis of laparoscopic versus open restorative proctocolectomy. Colorectal Dis 15:e340–e351
Ahmed Ali U, Keus F, Heikens JT et al (2009) Open versus laparoscopic (assisted) ileo pouch anal anastomosis for ulcerative colitis and familial adenomatous polyposis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006267.pub2
Tilney HS, Lovegrove RE, Heriot AG et al (2007) Comparison of short-term outcomes of laparoscopic versus open approaches to ileal pouch surgery. Int J Colorectal Dis 22:531–542
Causey MW, Stoddard D, Johnson EK et al (2013) Laparoscopy impacts outcomes favourably following colectomy for ulcerative colitis: a critical analysis of the ACS-NSQIP database. Surg Endosc 27:603–609
Bartels SA, Gardenbroek TJ, Ubbink DT et al (2013) Systematic review and meta-analysis of laparoscopic versus open colectomy with end ileostomy for non-toxic colitis. Br J Surg 100:726–733
Tajti J, Simonka Z, Paszt A et al (2015) Role of laparoscopic surgery in the treatment of ulcerative colitis; short- and mid-term results. Scand J Gastroenterol 50:406–412
Jimenez-Rodriguez RM, Diaz-Pavon JM, de Juan FDLP et al (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 28:815–821
Kim HJ, Choi GS, Park JS et al (2014) Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon’s experience. Dis Colon Rectum 57:1066–1074
Park IJ, Choi G, Lim K et al (2009) Multidimensional analysis of the learning curve for laparoscopic colorectal surgery: lessons from 1000 cases of laparoscopic colorectal surgery. Surg Endosc 23:839–846
Acknowledgements
The authors are pleased to acknowledge Professor Marc A Gladman (Academic Colorectal Unit, Sydney Medical School – Concord, University of Sydney, Australia) who provided methodological suggestions relating to the assessment of study quality and outcomes and manuscript edits.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Steven D Wexner has stock and stock options for consulting with Intuitive Surgical. The other authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent required as it is a systematic review of already published literature.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Renshaw, S., Silva, I.L., Hotouras, A. et al. Perioperative outcomes and adverse events of robotic colorectal resections for inflammatory bowel disease: a systematic literature review. Tech Coloproctol 22, 161–177 (2018). https://doi.org/10.1007/s10151-018-1766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-018-1766-5