Abstract
Background
Olanzapine is prescribed as prophylaxis for chemotherapy-induced nausea and vomiting at a dose of 2.5 or 5 mg in Asian countries. We compared the effectiveness of olanzapine 2.5 mg and 5 mg in preventing chemotherapy-induced nausea and vomiting among patients receiving high-emetogenic chemotherapy for lung cancer.
Methods
Using a Japanese national inpatient database, we identified patients who received olanzapine doses of 2.5 or 5 mg during high-emetogenic chemotherapy for lung cancer between January 2016 and March 2021. We conducted a 1:1 propensity score-matched analysis with adjustment for various factors, including those affecting olanzapine metabolism. The outcomes were additional antiemetic drug administration (within 2–5 days after chemotherapy initiation), length of hospital stay, and total hospitalization costs.
Results
Olanzapine 2.5 and 5.0 mg were used in 2905 and 4287 patients, respectively. The propensity score-matched analysis showed that olanzapine 2.5 mg administration was significantly associated with a higher proportion of additional antiemetic drug administration (36% vs. 31%, p < 0.001) than olanzapine 5 mg. The median length of hospital stay was 8 days in both groups. Total hospitalization cost did not differ significantly between the two doses of olanzapine (5061 vs. 5160 USD, p = 0.07). The instrumental variable analysis demonstrated compatible results.
Conclusion
Prophylactic use of olanzapine 2.5 mg during chemotherapy for lung cancer was associated with a higher rate of additional antiemetic drugs than olanzapine 5 mg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a major side effect of chemotherapy and affects patients' quality of life [1, 2]. CINV is common with high-emetogenic chemotherapy (HEC). HEC often requires a combination of three antiemetic drugs: a neurokinin 1 (NK1) receptor antagonist, a 5-hydroxytryptamine (5-HT3) receptor antagonist, and dexamethasone. However, half of patients receiving HEC in a previous study developed CINV due to the insufficient effectiveness of the three-drug combination [3].
Recent studies have demonstrated the effectiveness of olanzapine. Accordingly, the American Society of Clinical Oncology, National Comprehensive Cancer Network, and Multinational Association of Supportive Care in Cancer recommend the use of olanzapine as CINV prophylaxis for patients receiving HEC [4,5,6]. Furthermore, compared to the three-drug combination, olanzapine is cost-effective for patients with nausea and vomiting [7, 8].
Olanzapine 10 mg/day is safely used in the United States (US). However, due to the risk of oversedation, olanzapine 5 mg is used in Asian countries [9,10,11,12]. Furthermore, olanzapine 2.5 mg is prescribed for patients receiving HEC in real-world settings in Asian countries to avoid sleepiness. Previous retrospective studies have reported the effectiveness and safety of olanzapine 2.5 mg [13]. Although olanzapine 2.5 mg has not yet been approved as an antiemetic, clinicians may frequently prescribe it, considering its side effects (such as sleepiness). Specifically, several randomized controlled trials were conducted in Asian countries using olanzapine 2.5 mg [14, 15]. However, these previous studies had limitations, such as small sample sizes (n = 112 and 275) and lack of investigation of factors that influence olanzapine metabolism, such as smoking and drugs that induce cytochrome P450 enzymes [16,17,18,19,20]. A recent study has shown the effectiveness of olanzapine through subgroup analysis of risk factors for CINV [21]. However, no study has investigated factors affecting olanzapine metabolism. Additionally, previous studies of olanzapine 5 mg had a small proportion of patients aged ≥ 75 years. Therefore, the effectiveness and safety of olanzapine in the elderly remain unclear [9].
The optimal dose of olanzapine used as CINV prophylaxis remains unclear in Asian patients, particularly in the elderly. Therefore, we compared the effectiveness of two olanzapine doses (2.5 and 5 mg), using a nationwide inpatient database in Japan.
Patients and methods
Data source
This retrospective cohort study used patient data from the Diagnosis Procedure Combination database. As of November 2022, this nationwide database contained hospital administrative claims data and discharge abstracts of approximately 8,000,000 inpatients from more than 1200 acute care hospitals[22]. Participation in this database is compulsory for academic hospitals and voluntary for community hospitals. The database contains the following information: patient’s age, sex, body height, body mass index (BMI), smoking history, primary diagnosis, comorbidities at admission (International Classification of Diseases [ICD]-10 code), prescription information, tumor–node–metastasis stage of malignant tumor, length of hospital stay, discharge status, activities of daily living (ADL) following hospitalization at admission and discharge, and total hospitalization costs. The recorded diagnoses in the database were validated; for example, the specificity of lung cancer diagnosis was 96.7%, while the sensitivity was 50–80%. The specificity and sensitivity of the recorded procedures exceeded 90% [23, 24].
Patient selection
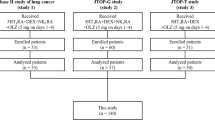
We identified inpatients who received cisplatin- or carboplatin-based chemotherapy for lung cancer (ICD-10 code: C34) and olanzapine 2.5 or 5 mg as CINV prophylaxis between January 2016 and March 2021. Prescription of olanzapine at chemotherapy initiation or before was regarded as prophylactic administration of olanzapine. We excluded patients (i) below 18 years old, (ii) with schizophrenia (F20, F22–25, F28, F29), (iii) with diabetes (F10–14) or who received treatment with insulin or oral hypoglycemic agents, or (iv) who received olanzapine for more than two days before chemotherapy or on day ≥ 5 after chemotherapy initiation, assuming that it could be for other purposes such as treatment for general anorexia, cachexia, and psychiatric symptoms [25, 26]. We divided the eligible patients into two groups: patients who were prescribed olanzapine 2.5 mg/day (the 2.5-mg olanzapine group) or olanzapine 5 mg/day (the 5-mg olanzapine group). The initial day of chemotherapy was defined as day 1.
The primary outcome was defined as additional antiemetic drug administration within the overall assessment period (days 2–5) and on each day (2, 3, 4, and 5). We considered the administration as a surrogate for CINV symptoms [9, 27, 28]. According to the antiemetics guidelines and common treatments in Japanese cancer hospitals, metoclopramide, domperidone, lorazepam, alprazolam, haloperidol, chlorpromazine, and prochlorperazine were considered additional antiemetic drugs [29, 30]. The secondary outcomes were dexamethasone use within days 2–5, length of hospital stay, and total hospitalization costs. One US dollar (USD) was equivalent to 110 Japanese yen.
This study was approved by the Institutional Review Board of the University of Tokyo (approval number 3501-5; May 19, 2021). The review board waived the requirement for patient-informed consent because of the use of anonymous data.
Covariates
We examined the following patients’ characteristics: sex, age, BMI, smoking index (0/1–19/ ≥ 20 pack-years), Charlson comorbidity index [31], Parkinson’s disease, independence in ADL, and cancer stage. The cancer stage was made according to the TNM classification. Age was categorized into four groups: < 64, 65–74, 75–84, and ≥ 85 years. There were four BMI groups: underweight, < 18.5; normal weight, 18.5–24.9; overweight, 25.0–29.9; and obese, ≥ 30 kg/m2. Comorbidities were assessed using the Charlson comorbidity index and categorized into 2, 3, 4, or ≥ 5. We also categorized treatment history into radiotherapy, chemotherapy regimen (Supplementary Table 1), antiemetic regimen according to the guidelines of the American Society of Clinical Oncology and National Comprehensive Cancer Network (Supplementary Table 2), use of olanzapine-interacting drugs, number of chemotherapy cycles (one, two, three, or above three), support from a palliative care team, emergency admission, types of hospitals (teaching hospital or not), and fiscal year of admission. Regarding olanzapine-interacting drugs, we included corticosteroids (except dexamethasone), hypnotics (benzodiazepines, non-benzodiazepines, and other hypnotic drugs), barbiturates, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, noradrenergic and specific serotonergic antidepressants, tricyclic antidepressants, tetracyclic antidepressants, other antidepressants, multi-acting receptor-targeted antipsychotic drugs (except for olanzapine 2.5 and 5 mg), serotonin-dopamine antagonists, dopamine receptor antagonists (phenothiazines, butyrophenones, and benzamides), anti-parkinsonian drugs (levodopa, levodopa-carbidopa, levodopa-benserazide, ropinirole, pramipexole, and rotigotine), carbamazepine, omeprazole, and rifampicin [16, 17, 32, 33]. We identified patients who received these medications within 7 days before chemotherapy initiation.
Statistical analysis
We performed a propensity score analysis to adjust for confounding by indication and to compare the outcomes between both groups. A propensity score analysis can effectively adjust for measured confounders and is used to balance patients’ backgrounds in a retrospective study [34]. We conducted propensity score matching at a 1:1 ratio. Propensity scores were calculated with a logistic regression model using the above-mentioned patient and treatment variables as covariates. Each patient who received olanzapine 2.5 mg was matched with a patient who received olanzapine 5 mg using the nearest-neighbor matching method without replacement. The caliper width was ≤ 0.2 of the pooled standard deviation of estimated logits of the propensity score. We calculated standardized differences to examine the balance in baseline covariates of patients between both groups. An absolute standardized difference below 10% denoted a negligible difference between both groups [35]. Continuous and categorical variables were compared using the t test and Chi square test, respectively.
We also performed the four subgroup analyses for the primary outcome. Olanzapine metabolism is reportedly strongly affected by sex, age, and smoking [16, 18,19,20]; therefore, we evaluated patients in the following subgroups: sex, age (< 65 or ≥ 65 years), and with and without a smoking history. Additionally, we evaluated the data of patients who received only a cisplatin regimen.
Finally, we conducted two sensitivity analyses to confirm the robustness of our results. First, we only included patients who were using three antiemetic drugs (a 5-HT3 receptor antagonist, an NK1 receptor antagonist, and dexamethasone). Second, we conducted an instrumental variable analysis to address unmeasured confounders. Facility proportion of annual 2.5 mg olanzapine usage was an instrumental variable because facility treatment proportion is the best-known instrumental variable type [36, 37]. We used a two-stage residual inclusion estimation framework with robust standard errors, using background patient and treatment characteristics as covariates [38]. An F-statistics of > 10 indicated that our instrumental variable was highly correlated with treatment using additional antiemetic drugs. The first stage was a generalized linear model adjusted for the patient and treatment backgrounds. This model was used to measure the association between the facility proportion of annual olanzapine 2.5 mg usage and our instrument. From this model, we determined the raw residual for each patient by calculating the difference between the model-predicted probability of facility proportion of annual olanzapine 2.5 mg usage and olanzapine 2.5 mg administration. The residuals were then included as an additional covariate in the second-stage model. In the second-stage binomial regression model adjusted for patient and treatment backgrounds used in the first stage and the residual calculated in the first stage, we estimated the association between olanzapine 2.5 or 5 mg administration and additional antiemetic drugs administration. We then calculated a risk difference for the primary outcome (i.e., additional antiemetic drug administration within the overall assessment period) in the olanzapine 2.5 mg group and compared it with that of the olanzapine 5 mg group. To demonstrate the quasi-randomization of treatment assignments by the instrumental variable, we described the patients’ characteristics according to the mean value of the instrumental variable. All analyses were performed using Stata/SE 17.0 software (StataCorp, College Station, TX, USA). Continuous variables are presented as median and interquartile range (IQR). Categorical variables are expressed as numbers and percentages. All reported p values are two-sided, and p < 0.05 was considered statically significant.
Results
We identified 17,883 eligible patients. We excluded 10,691 patients who met the exclusion criteria: (i) four patients were < 18 years old; (ii) 1202 patients had schizophrenia; (iii) 999 had diabetes or used hypoglycemic drugs; and (iv) 8486 received olanzapine two days before chemotherapy or 5 days after chemotherapy. Of the 7192 included patients, 2905 (40%) and 4287 (60%) patients comprised the olanzapine 2.5 and 5 mg groups, respectively (Fig. 1). After 1:1 propensity score matching, each group included 2619 patients; the background characteristics were comparable between the two groups.
Tables 1 and 2 show the background characteristics before and after propensity score matching, respectively. Before the propensity score matching, patients in the olanzapine 2.5 mg group were more likely to be female, older, have a lower BMI, a higher comorbidity index, lower smoking index, and to have received multiple chemotherapy sessions than those in the olanzapine 5 mg group. These backgrounds were well balanced after propensity score matching.
Table 3 shows the outcomes of all the patients and those of the 1:1 propensity score-matched patients. Before matching, the olanzapine 2.5 mg group had a higher proportion of patients with primary outcomes within the overall assessment period (days 2–5) (36% vs. 28%). The propensity score-matched analysis showed that the olanzapine 2.5 mg group was associated with a higher proportion of additional antiemetic drug administration within the overall assessment period (36% vs. 31%; difference, 6.2% [95% confidence interval, CI 3.7 to 8.7]) but a lower proportion of dexamethasone on days 2 (38% vs. 40%, difference, -1.9% [95% CI -4.5 to 0.7]), 3 (13% vs. 16%; difference, -3.3% [95% CI -5.2 to -1.4]), and 4 (13% vs. 15%; difference, -2.1% [95% CI -3.9 to -0.2], when compared to the olanzapine 5 mg group. The median length of hospital stay was not significantly different between both groups (8 vs. 8 days; difference, 0 days [95% CI -0.5 to 0.7]). Total hospitalization costs were not significantly different between both groups (5061 vs. 5160 USD; difference, -108 USD [95% CI -396 to 180]).
The subgroup analyses showed similar results to those of analyses of the 1:1 propensity score-matched cohort (Tables 4, 5). In the subgroups of male sex, age ≥ 65 years, and smoker status, the proportion of patients who received additional antiemetics was significantly higher in the olanzapine 2.5 mg group than in the olanzapine 5 mg group. No significant difference was observed in the female sex and age < 65 years subgroups. The patients’ background characteristics categorized according to the mean value of the instrumental variable are shown in Supplemental Tables 3 and 4. The olanzapine 2.5 mg group was significantly associated with higher use of additional antiemetic drugs within the overall assessment period (risk difference, 0.076 [95% CI 0.055 to 0.10]). Sensitivity analysis for patients using three antiemetic drugs demonstrated consistent results (Supplemental Table 5).
Discussion
In this study, using a nationwide database in Japan, we compared the outcomes of olanzapine 2.5 mg and 5 mg in terms of CINV prophylaxis. Olanzapine 2.5 mg administration was associated with a higher proportion of patients using additional antiemetics than olanzapine 5 mg. However, in the female sex or age < 65 years subgroups, the proportion of use of additional antiemetics in the olanzapine 2.5 mg group was not significantly different to that in the olanzapine 5 mg group. The length of hospital stay was not significantly different between both groups.
The universal healthcare insurance system in Japan began to cover the cost of olanzapine used as CINV prophylaxis in June 2017. The rate of olanzapine administration as CINV prophylaxis in Japan has increased to that in the US [12, 30]. Our study showed that olanzapine 2.5 mg was administered to mainly females, elderly patients, and patients who received chemotherapy multiple times. These differences were adjusted for in our propensity score analyses.
The olanzapine 2.5 mg group needed additional antiemetics more frequently than the 5 mg group, especially within days 2–4. Our results are consistent with those of randomized controlled trials of olanzapine 5 mg used as CINV prophylaxis; these trials recorded similar trends of additional antiemetics administration [9, 27]. Patients who receive HEC are likely to experience severe CINV on days 2–5 [39]. The relationship between plasma concentration of olanzapine and dose is linear; therefore, the efficacy of olanzapine 2.5 mg may be inferior to that of 5 mg in CINV [16]. In this study, the length of hospital stay and total hospitalization costs did not differ between both groups. These outcomes could have been affected by the side effects of olanzapine 5 mg such as sleepiness or somnolence.
A previous study of patients with breast cancer showed the efficacy and safety of olanzapine 2.5 mg for CINV [13]. However, the report included only few patients (n = 45) with breast cancer. Consequently, the patient’s characteristics that may affect the efficacy of olanzapine (e.g., sex, age, smoking history, and olanzapine-interacting drugs use) were not adjusted in the analysis [16, 17]. Additionally, the study did not compare the outcomes between olanzapine 2.5 and 5 mg administration. Contrarily, our study showed the advantageous effectiveness of olanzapine 5 mg over 2.5 mg by adjusting patient characteristics using a nationwide database.
This is the first study to explore the relationship between factors affecting the metabolism of olanzapine and the antiemetic efficacy of varying dosages. A difference was found in the subgroups of male sex and age ≥ 65 years. However, a previous randomized trial showed no difference between olanzapine 5 mg and placebo groups by sex or age [21]. The results in our study differ from those in the previous study. Our study used retrospective data, and nausea was assessed solely based on an antiemetic prescription. Therefore, nausea that required no antiemetics may not have been adequately evaluated. We analyzed the effect of smoking history in subgroup analysis. Some studies also reported that the increased clearance of olanzapine due to smoking needs a few days to disappear after smoking cessation [18, 20, 40].Thus, the proportion of additional antiemetics administration did significantly differ in a subgroup with smokers at admission.
Propensity score analysis has advantages compared with traditional multivariable regression in that it can eliminate measured confounding by balancing the confounders and directly estimate the treatment effect. However, propensity score analysis has a limitation in that it cannot adjust for unmeasured confounders. In the present study, unmeasured confounders (e.g., brain metastasis, dosage of cisplatin or carboplatin, and previous history of nausea) may have influenced the olanzapine doses. We therefore conducted instrumental variable analysis, which can theoretically adjust even for unmeasured confounders. The results of the instrumental variable analysis, which accounted for unmeasured background factors, supported our findings of the main analysis (1:1 propensity score analysis).
This study has several limitations. First, the CINV symptoms could have been underestimated as the database does not contain patient-reported outcomes; we used additional antiemetics administration as an alternative to CINV symptoms. Therefore, we could only investigate patients with severe nausea or vomiting. Previous trials have reported that nausea occurred in 40% of patients who receive olanzapine 5 mg after chemotherapy. In this study, the proportion of patients with additional antiemetics was 22% [9]. However, because the underestimation would have occurred equally in both groups, it would not have skewed our results. Additionally, we could not evaluate outcomes in the outpatient settings after discharge because we only investigated the additional antiemetics administered during hospitalization. However, the underestimation of outcomes was small because patients in this study were hospitalized for approximately one week. Second, we were able to evaluate the prescription but not the actual administration of olanzapine. Patients in the olanzapine 5 mg group may have stopped olanzapine due to the side effects (e.g., sleepiness in the daytime) [9,10,11]. Nevertheless, the olanzapine 5 mg group received significantly fewer additional antiemetics than the olanzapine 2.5 mg group. Therefore, the results may have not been altered by the lack of information on actual olanzapine administration. Third, we compared between the groups using propensity score-matched analysis. Although we generated 2626 pairs for analysis, we lost approximately 1800 patients. Moreover, propensity score analysis is not able to adjust for unmeasured confoundings. However, we adjusted for more than 40 measured confoundings including type of drugs, chemotherapy regimen, and antiemetics regimens. There were few unmeasured confoundings in our study. Fourth, although the number of patients using three antiemetic drugs was small, the results in the sensitivity analysis, which only included patients who received all three antiemetic drugs, were similar to those in the main results.
Conclusion
Olanzapine 2.5 mg administration for CINV prophylaxis was significantly associated with additional antiemetics administration. However, among female patients or patients aged < 65 years, the outcomes of olanzapine 2.5 mg was not significantly different from those in the olanzapine 5 mg group—in terms of additional antiemetics administration.
References
Sommariva S, Pongiglione B, Tarricone R (2016) Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: a systematic review. Crit Rev Oncol Hematol 99:13–36. https://doi.org/10.1016/j.critrevonc.2015.12.001
Fernández-Ortega P, Caloto MT, Chirveches E et al (2012) Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer 20(12):3141–3148. https://doi.org/10.1007/s00520-012-1448-1
Matsumoto K, Takahashi M, Sato K et al (2020) A double-blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy-induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide. Cancer Med 9(10):3319–3327. https://doi.org/10.1002/cam4.2979
Hesketh PJ, Kris MG, Basch E et al (2020) Antiemetics: ASCO guideline update. J Clin Oncol 38(24):2782–2797. https://doi.org/10.1200/JCO.20.01296
Berger MJ, Ettinger DS, Aston J et al (2017) Antiemesis, version 2.2017 featured updates to the NCCN guidelines. JNCCN J Nat Compr Cancer Network 15:883–893
Herrstedt J, Roila F, Warr D et al (2017) 2016 updated MASCC/ESMO consensus recommendations: Prevention of nausea and vomiting following high emetic risk chemotherapy. Support Care Cancer 25(1):277–288. https://doi.org/10.1007/s00520-016-3313-0
Chanthawong S, Lim YH, Subongkot S et al (2019) Cost-effectiveness analysis of olanzapine-containing antiemetic therapy for managing highly emetogenic chemotherapy in Southeast Asia: a multinational study. Support Care Cancer 27(3):1109–1119. https://doi.org/10.1007/s00520-018-4400-1
Chow R, Chiu L, Herrstedt J et al (2021) Cost-effectiveness analysis of olanzapine-containing antiemetic therapy for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) in highly emetogenic chemotherapy (HEC) patients. Support Care Cancer 29(8):4269–4275. https://doi.org/10.1007/s00520-020-05977-x
Hashimoto H, Abe M, Tokuyama O et al (2020) Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21(2):242–249. https://doi.org/10.1016/S1470-2045(19)30678-3
Ithimakin S, Theeratrakul P, Laocharoenkiat A et al (2020) Randomized, double-blind, placebo-controlled study of aprepitant versus two dosages of olanzapine with ondansetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving high-emetogenic chemotherapy. Support Care Cancer 28(11):5335–5342. https://doi.org/10.1007/s00520-020-05380-6
Chow R, Navari RM, Terry B et al (2022) Olanzapine 5 mg vs 10 mg for the prophylaxis of chemotherapy-induced nausea and vomiting: a network meta-analysis. Support Care Cancer 30(2):1015–1018. https://doi.org/10.1007/s00520-021-06606-x
Childs DS, Helfinstine DA, Sangaralingham L et al (2022) Slow uptake of an effective therapy: patterns of olanzapine prescribing for those receiving highly emetogenic chemotherapy. JCO Oncol Pract 18(12):e1953–e1960. https://doi.org/10.1200/OP.22.00389
Sato J, Kashiwaba M, Komatsu H et al (2016) Effect of olanzapine for breast cancer patients resistant to triplet antiemetic therapy with nausea due to anthracycline-containing adjuvant chemotherapy. Jpn J Clin Oncol 46(5):415–420. https://doi.org/10.1093/jjco/hyw011
Sandhya L, Devi Sreenivasan N, Goenka L et al (2023) Randomized double-blind placebo-controlled study of olanzapine for chemotherapy-related anorexia in patients with locally advanced or metastatic gastric, hepatopancreaticobiliary, and lung cancer. J Clin Oncol 41(14):2617–2627. https://doi.org/10.1200/JCO.22.019971
Bajpai J, Kapu V, Rath S et al (2024) Low-dose versus standard-dose olanzapine with triple antiemetic therapy for prevention of highly emetogenic chemotherapy-induced nausea and vomiting in patients with solid tumours: a single-centre, open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol 25(2):246–254. https://doi.org/10.1016/S1470-2045(23)00628-9
Callaghan JT, Bergstrom RF, Ptak LR et al (1999) Olanzapine pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet 37(3):177–193. https://doi.org/10.2165/00003088-199937030-00001
Lucas RA, Gilfillan DJ, Bergstrom RF (1998) A pharmacokinetic interaction between carbamazepine and olanzapine: observations on possible mechanism. Eur J Clin Pharmacol 54(8):639–643. https://doi.org/10.1007/s002280050527
Lowe EJ, Ackman ML (2010) Impact of tobacco smoking cessation on stable clozapine or olanzapine treatment. Ann Pharmacother 44(4):727–732. https://doi.org/10.1345/aph.1M398
Gex-Fabry M, Balant-Gorgia AE, Balant LP (2003) Therapeutic drug monitoring of olanzapine: the combined effect of age, gender, smoking, and comedication. Ther Drug Monit 25(1):46–53. https://doi.org/10.1097/00007691-200302000-00007
An H, Fan H, Chen S et al (2021) Effects of dose, age, sex, body weight, and smoking on plasma concentrations of olanzapine and N-desmethyl olanzapine in inpatients with schizophrenia. J Clin Psychopharmacol 41(3):255–259. https://doi.org/10.1097/JCP.0000000000001390
Abe M, Yamaguchi T, Fujita Y et al (2023) Efficacy of olanzapine in addition to standard triplet antiemetic therapy for cisplatin-based chemotherapy: a secondary analysis of the J-FORCE randomized clinical trial. JAMA Netw Open. https://doi.org/10.1001/jamanetworkopen.2023.10894
Yasunaga H (2019) Chapter I real world data in Japan: chapter I NDB. ACE 1(2):28–30
Yamana H, Moriwaki M, Horiguchi H et al (2017) Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 27(10):476–482. https://doi.org/10.1016/j.je.2016.09.009
Shigemi D, Morishima T, Yamana H et al (2021) Validity of initial cancer diagnoses in the diagnosis procedure combination data in Japan. Cancer Epidemiol. https://doi.org/10.1016/j.canep.2021.102016
Naing A, Dalal S, Abdelrahim M et al (2015) Olanzapine for cachexia in patients with advanced cancer: an exploratory study of effects on weight and metabolic cytokines. Support Care Cancer 23(9):2649–2654. https://doi.org/10.1007/s00520-015-2625-9
Sandhya L, Nirmala SD et al (2023) Randomized double-blind placebo-controlled study of olanzapine for chemotherapy-related anorexia in patients with locally advanced or metastatic gastric, hepatopancreaticobiliary, and lung cancer. J Clin Oncol. https://doi.org/10.1200/JCO.22
Navari RM, Qin R, Ruddy KJ et al (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375(2):134–142. https://doi.org/10.1056/NEJMoa1515725
Kunitomi Y, Nakashima M, Seki T et al (2021) Intergenerational comparison of 5-HT 3 RA in the prevention of chemotherapy-induced nausea and vomiting in gastric cancer patients receiving cisplatin-based chemotherapy: an observational study using a Japanese administrative claims database. Support Care Cancer 29(7):3951–3959. https://doi.org/10.1007/s00520-020-05958-0
Aogi K, Takeuchi H, Saeki T et al (2021) Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for antiemesis. Int J Clin Oncol 26(1):1–17. https://doi.org/10.1007/s10147-020-01818-3
Tamura K, Aiba K, Saeki T et al (2017) Breakthrough chemotherapy-induced nausea and vomiting: report of a nationwide survey by the CINV Study Group of Japan. Int J Clin Oncol 22(2):405–412. https://doi.org/10.1007/s10147-016-1069-7
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Sun L, McDonnell D, Yu M et al (2019) A Phase I open-label study to evaluate the effects of rifampin on the pharmacokinetics of olanzapine and samidorphan administered in combination in healthy human subjects. Clin Drug Investig 39(5):477–484. https://doi.org/10.1007/s40261-019-00775-8
Nozawa M, Ohnuma T, Matsubara Y et al (2008) The relationship between the response of clinical symptoms and plasma olanzapine concentration, based on pharmacogenetics: Juntendo University schizophrenia projects (JUSP). Ther Drug Monit 30(1):35–40. https://doi.org/10.1097/FTD.0b013e31816336fd
Rosenbaum PR, Rubin DB (1985) Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 39(1):33–38. https://doi.org/10.1080/00031305.1985.10479383
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
Baiocchi M, Cheng J, Small DS (2014) Instrumental variable methods for causal inference. Stat Med 33(13):2297–2340. https://doi.org/10.1002/sim.6128
Aso S, Yasunaga H (2020) Introduction to instrumental variable analysis. Ann Clin Epidemiol 2(3):69–74
Terza JV, Basu A, Rathouz PJ (2008) Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ 27(3):531–543. https://doi.org/10.1016/j.jhealeco.2007.09.009
Hesketh PJ, Van Belle S, Aapro M et al (2003) Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer 39(8):1074–1080. https://doi.org/10.1016/S0959-8049(02)00674-3
Chui CY, Taylor SE, Thomas D et al (2019) Prevalence and recognition of highly significant medication-smoking cessation interactions in a smoke-free hospital. Drug Alcohol Depend 200:78–81. https://doi.org/10.1016/j.drugalcdep.2019.03.006
Funding
Open Access funding provided by The University of Tokyo. This work was supported by grants from the Ministry of Health, Labor and Welfare, Japan (23AA2003 and 22AA2003).
Author information
Authors and Affiliations
Contributions
HS, TK, and TJ conceived the study concept and study design. SA, KM, and HM performed the data compilation and synthesis. HM, KF, and HY processed the corrected data. HC, TK, and SA carried out statistical analysis. HC drafted the manuscript. TK, SA, TJ, and HY revised the manuscript. HY supervised the research project. All authors participated in interpreting the results and writing the report. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Konishi received grants from Pfizer Co. Ltd., the Japan Kampo Medicines Manufacturers’ Association, and Kanzawa Medical Research Foundation outside the submitted work. The authors declare no conflict of interest.
Ethical approval
Authorization was obtained from the Institutional Review Board of the University of Tokyo (approval number 3501-5; May 19, 2021). This study has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
The requirement for informed consent was waived due to the use of anonymous data.
Consent to publish
The requirement for consent to publish was waived due to the use of anonymous data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Suzuki-Chiba, H., Konishi, T., Aso, S. et al. Comparison of olanzapine 2.5 mg and 5 mg in the prevention of chemotherapy-induced nausea and vomiting: a Japanese nationwide database study. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02603-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02603-2