Abstract
With the development of poly(ADP-ribose) polymerase inhibitors, the treatment of advanced ovarian cancer is changing dramatically. The purpose of this narrative review is to provide a direction for the individualization of advanced ovarian cancer treatment based on the mechanism of action of molecularly targeted drugs currently used in Japan. The PAOLA-1 study showed very good progression-free survival in patients with homologous recombination deficiency tumors who underwent complete surgery with primary debulking surgery and who received olaparib plus bevacizumab. Niraparib has high intratumor penetration, and in a subgroup analysis of the PRIMA study, it was most effective in patients with residual tumors after interval debulking surgery. These data suggest the importance of achieving complete surgery and aiming for cure in the treatment of ovarian cancer and how the use of bevacizumab, olaparib, and niraparib should be individualized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advanced ovarian cancer has long been regarded as a fatal disease. In recent years, however, development of therapies for advanced ovarian cancer, including Poly(ADP-ribose) polymerase (PARP) inhibitors, such as olaparib (Ola) and niraparib (Nira), has progressed to the point at which a complete cure is now possible in a significant number of patients. In Japan, these two PARP inhibitors have been approved by healthcare insurance. However, many gynecological oncologists seem to be confused about how to choose between Ola, Nira, and bevacizumab (Bev) in advanced ovarian cancer. Essentially, to decide which treatment is superior in a scientifically sound manner, it is necessary to conduct head-to-head Phase III clinical trials led by researchers who are independent of the pharmaceutical industry with the primary endpoint set as a comparison of different drugs. For example, in the development of a treatment for coronavirus 2019 (COVID-19), the World Health Organization took the lead and conducted such a trial [1]. However, deciding whether Ola or Nira is better for ovarian cancer is not an urgent public health issue, and it is not realistic to expect public institutions to provide large amounts of funding to set up phase III clinical trials for this purpose. The decision of gynecological oncologists to stop thinking about whether to use Ola, Nira, or Bev because of the lack of scientifically sound data does not lead to patient-centered medicine. To provide patient-centered care, gynecological oncologists need to understand as much as possible about data concerning Ora, Nira, and Bev, including mechanisms of action and subgroup analysis of clinical trials. This paper is written as a narrative review because systematic reviews and meta-analyses are difficult to conduct when attempting to examine data from various angles in a situation in which insufficient evidence is found. This paper mainly discusses PARP inhibitors and surgery in primary treatment, and Bev is reviewed in detail in another paper [2].
The detection of germline or somatic BRCA1/2 mutations (gBRCA1/2 or sBRCA1/2 mutations) and DNA homologous recombination deficiency (HRD) is important for selecting patients who will benefit from PARP inhibitor therapy. The BRACAnalysis® diagnostic system tests for the presence of gBRCA1/2 mutations while the myChoice® diagnostic system tests for the presence of tumor BRCA1/2 mutations (tBRCA1/2 mutations; gBRCA1/2 mutations + sBRCA1/2 mutations) or HRD score (genomic instability score; GIS) ≥ 42. These tests have already been approved by the Japanese healthcare insurance.
Biological properties and drug sensitivity of ovarian cancer vary greatly depending on the histological type, and treatment strategies also differ. The most common histological type of ovarian cancer is high-grade serous carcinoma (HGSC), and the most advanced stage III/IV ovarian cancer is HGSC. Therefore, this paper will focus on HGSC unless otherwise stated.
Insurance approval of molecularly targeted drugs for the first-line treatment of ovarian cancer in Japan
In Japan, molecularly targeted drugs have been approved for the first-line treatment of The International Federation of Gynecology and Obstetrics (FIGO) stage III–IV ovarian cancer as follows:
-
In 2013, paclitaxel and carboplatin (TC) chemotherapy with Bev in combination and maintenance therapy was approved.
-
In 2018, maintenance therapy with Ola was approved for patients who have tumors with germline or somatic BRCA mutations and have had a response to platinum-based chemotherapy.
-
In 2020, maintenance therapy with Nira was approved for patients who have responded to platinum-based chemotherapy with or without BRCA mutations or HRD.
-
In 2021, maintenance therapy with Ola and Bev was approved for patients with tumors that have HRD and have responded to platinum-based chemotherapy with Bev.
PARP inhibitors; Olaparib (Ola) and Niraparib (Nira)
Comparison of Ola and Nira in terms of mechanisms of action
Originally, the mechanism of action of PARP inhibitors was explained by the concept of “synthetic lethality”. In other words, PARP plays an important role in single-strand break repair, and PARP inhibitors cause an inhibition of single-stranded DNA break repair, resulting in double-stranded DNA breaks, which are repaired by the HRR pathway. However, in HRD tumor cells, which cannot repair double-stranded DNA breaks via the HRR pathway, the non-homologous end joining pathway is used, resulting in the accumulation of genetic mutations and cell death [3]. Although this finding may be true, it is difficult to explain the phenomenon of cell death at 24–48 h after the addition of PARP inhibitors in vitro as being based on the accumulation of genetic mutations. Later, it was reported that PARP trapping, in which PARP is attached to DNA, is the main mechanism of cytotoxicity induced by PARP inhibitors [4]. Since the HRR pathway is mainly used to repair DNA damage caused by PARP trapping, PARP inhibitors cause cell death in HRD cells. However, even in HR proficient (HRp) cells with preserved HRR capacity, continuous exposure of highly proliferative cells to high concentrations of PARP inhibitors results in cell death due to the lack of capability to repair DNA damage caused by PARP trapping [37].
The in vitro cytotoxicity potential of Ola and Nira varies among reports but does not seem to be significantly different. In the first paper comparing their PARP trapping capability, Nira was found to have a slightly stronger PARP trapping capability [4], but in a later report, data showed that Ola had a stronger PARP trapping capability [5]. In addition, PARP trapping is caused by allosteric changes in the structure of PARP, and from this point of view, Nira has a molecular structure that is more likely to dissociate PARP from DNA than Ola [6]. The differences in the strength of cytotoxicity and PARP trapping between Ola and Nira in different reports may be due to differences in the cells and experimental systems used, and these differences cannot explain the differences in the efficacy of Ola and Nira in clinical trials.
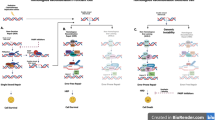
The major difference between Ola and Nira is the in vivo distribution of the drug. The steady-state plasma concentrations of PARP inhibitors in humans show that Ola fluctuates between 3 and 8 μg/mL every 12 h at a dose of 300 mg bid [7], while Nira fluctuates between 0.6 and 0.8 μg/mL every 24 h at a dose of 200 mg qd [8] as shown in Fig. 1. Thus, compared to Ola, Nira is immediately transferred from plasma into tissues, resulting in lower plasma concentrations and less fluctuations. In the immunodeficient mouse model in which tumor cells were inoculated, the concentration of Nira in the tumor tissue was shown to be higher than that in the plasma, whereas the concentration of Ola in the plasma was higher than that in the tumor tissue [9] as shown in Table 1. In this mouse model, Nira was also detected in the bone marrow, whereas Ola was not detected in the bone marrow.
The ABCB1 gene encodes an active transport protein called MDR1 or P-glycoprotein, which is physiologically expressed in the brain, placenta, and testis and prevents accumulation of toxins. The SLC25A-ABCB1 fusion gene is found in about 10% of chemo-resistant recurrent ovarian cancers, and tumors carrying the fusion gene express high levels of ABCB1 [10]. ABCB1 expression in cancer cells results in resistance to its substrates, taxanes, doxorubicin, and etoposide [11]. In addition, among PARP inhibitors, Ola and rucaparib are susceptible to high ABCB1 expression, while veliparib is not affected [12]. Although Nira is also a substrate of ABCB1, it is relatively less affected by high ABCB1 expression than Ola due to its higher penetration into tumor cells. In fact, Nira can cross the blood–brain barrier in which ABCB1 is highly expressed, and Nira has been reported to respond to brain metastasis of BRCA-mutant tumors [13, 14].
Because Ola has a high concentration in plasma, while its intratumor concentration is lower than that in plasma, Ola is more effective when it does not form a tumor mass. Because the blood and intratumor concentrations of Ola fluctuate, the therapeutic range is not achieved for HRp tumor cells for a long period of time, making it difficult to achieve an effect on HRp tumor cells. However, Ola has very low distribution in the bone marrow, so myelosuppression is mild even in the therapeutic range. On the other hand, Nira is effective even in the presence of a tumor mass, and because of its low concentration variation, it is thought to take a long time to reach the therapeutic range even for HRp tumor cells. In the NOVA study, which examined the effect of Nira as maintenance therapy for platinum-sensitive relapse, the HR for gBRCA wild-type tumors was 0.58 in patients who had complete responses (CR) on prior therapy compared with 0.35 in patients who had partial responses (PR) [15]. This data reflects the good tissue penetration of Nira. However, at the doses at which Nira exerts its antitumor effect, myelosuppression is more severe.
Ola at first line treatment; SOLO1 and PAOLA-1 studies
In the SOLO1 study, patients with BRCA1/2 mutations were treated with maintenance Ola for 2 years without Bev. In the long-term follow-up data, the 5-year progression-free survival (PFS) was 56% in the group who underwent complete surgery (R0) by primary debulking surgery (PDS) and 42% in the high-risk group other than PDS R0 [16]. Thus, although the survival rate is better with PDS R0, high-risk patients may still be able to survive, and the data underscores the importance of PARP inhibitors in BRCA-mutated cases. In the SOLO1 study, the HR was 0.33 for patients with no residual tumor at surgery (PDS R0 + interval debulking surgery (IDS) R0) and 0.44 for patients with residual tumor at surgery, and patients with no residual tumor at surgery responded better to Ola [17].
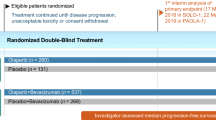
In the PAOLA-1 study, TC + Bev (Bev combination and maintenance) regimen was combined with Ola or placebo maintenance. Since Ola maintenance was ineffective in HRp patients [18], Ola maintenance combined with Bev was approved only in HRD-positive patients. Comparing single nucleotide polymorphism (SNP) array data of HGSC tumors before and after chemotherapy, we found that the GIS of the tumors decreased after chemotherapy [19]. Therefore, if IDS specimens after neoadjuvant chemotherapy (NAC) are submitted to the myChoice® test, it is likely that tumor BRCA mutations can be detected, but HRD-positive cases by GIS will be harder to detect.
Regarding with the patient backgrounds in the PAOLA-1 study (Fig. 2), surgical outcomes showed that PDS R0 was more common in BRCA-mutant and BRCA wild-type HRD-positive patients, and no surgery was more common in HRp patients. In terms of chemotherapy sensitivity, PR was slightly more common in HRp patients. Patients with PDS R0 or IDS R0 who did not become negative for CA125 after chemotherapy were included in PR. In the overall population, the HRs were 0.53 for no evidence of disease (NED), 0.44 for CR, and 0.86 for PR, indicating a clear difference in treatment effect before the start of Ola. This finding may be not only because of the slightly higher PR in HRp but also because of the poor drug penetration of Ola into the tumor mass.
Patient background of PAOLA-1 study [18]. BRCAm; BRCA mutation, BRCAwt; BRCA wild type
In terms of differences between BRCA1 and BRCA2 mutations, in the SOLO1 study, comparing Ola versus placebo, the 3-year PFS for the BRCA1 mutation group was found to be 55% versus 30% with an HR of 0.41 while the 3-year PFS for the BRCA2 mutation group was 85% versus 30% with an HR of 0.20; thus, the effects of Ola were higher in the BRCA2 mutations [17]. In the PAOLA-1 study, when comparing Ola + Bev versus placebo + Bev, the BRCA1 mutation group had a 3-year PFS of 65 versus 30% with an HR of 0.29, and the BRCA2 mutation group had a 3-year PFS of 85 versus 40% with an HR of 0.23, with slightly better results in the BRCA2 mutation group [20].
In the PAOLA-1 study, a subgroup analysis was performed in which patients were divided into a PDS R0 group and a non-PDS R0 group (high-risk group). In high-risk cases, the HRs for BRCA mutation and HRD (BRCA mutation + BRCA wild-type and GIS ≥ 42) with Ola were 0.37 and 0.39, respectively, whereas in PDS R0, the HRs for BRCA mutation and HRD were 0.11 and 0.15, respectively [21]. PFS2 in HRD cases also showed a large difference in the effect of Ola between the two groups with HR 0.66 in the high-risk group but HR 0.21 in the PDS R0 group [22].
Nira as first line treatment; PRIMA study
In the PRIMA study, PDS R0 was excluded, and only cases with responses to prior chemotherapy were enrolled to evaluate the effect of maintenance therapy with Nira compared to placebo. The risk of progression was HR 0.40 for BRCA-mutated cases, HR 0.50 for BRCA wild type and HRD positive, and HR 0.68 for HRp, all of which showed a significant effect of Nira treatment [23]. In a subgroup analysis, the association between surgical outcome and the effect of Nira was HR 0.41 for IDS R1 compared to HR 0.58 for PDS R1 and HR 0.65 for IDS R0 [24]. Thus, it is surprising that a subgroup with a HR of 0.41 was found when the selection is based on clinical information and not on HRD status. Although the PRIMA study was originally designed to evaluate the efficacy of Nira by enrolling patients with HRD using response to chemotherapy as a clinical biomarker, the highest efficacy was observed in patients with IDS R1 who were considered to respond poorly to chemotherapy. This result was unexpected.
In the PRIMA study, the HRs were 0.39 for BRCA1 mutations and 0.35 for BRCA2 mutations [25]. Thus, unlike Ola, there is not much difference between BRCA1 and BRCA2 mutations in first-line treatment. In the VELIA trial, veliparib was associated with HRs of 0.38 and 0.64 for BRCA1 and BRCA2 mutations, respectively, with the opposite trend [26]. The reason for this difference between PARP inhibitor types is currently unknown.
In the NOVA study of Nira maintenance therapy for platinum-sensitive recurrent ovarian cancer, when Nira was started at 300 mg/day, only about 50% of patients could be maintained at 300 mg/day after the second month and about 20% after the fifth month [27]. Subsequently, Nira clinical trials were conducted with individualized starting doses of 300 mg/day for patients with body weight ≥ 77 kg and platelet count ≥ 150,000/μl, and 200 mg/day for patients with body weight < 77 kg or platelet count < 150,000/μl. In the PRIMA study, the dose was switched from a fixed starting dose (Nira; n = 315, placebo; n = 169) to an individualized starting dose (Nira; n = 158, placebo; n = 86). At the fixed starting dose, the dose was reduced in 74.8% of patients with 75.9% of Grade 3 or higher adverse events, including 48.3% related to thrombocytopenia, and HR 0.59 for effect. At the individualized starting dose, the dose was reduced in 65.7% of patients, and adverse events improved to 60.4% of Grade 3 or higher adverse events, including 21.3% related to thrombocytopenia, but the effect was somewhat inferior at HR 0.69 [28]. In Japan, niraparib-2001 and niraparib-2002 studies were conducted with a fixed starting dose for platinum-sensitive relapse cases [29, 30], however, insurance approval was granted for individualized starting doses including first-line maintenance therapy.
Comparison of clinical trial data of Ola, Nira, and Bev by disease status
Comparison of Ola and Ola + Bev in BRCA mutation positive cases
In the high-risk group other than PDS R0, the 1-year PFS of the Ola + Bev arm of the PAOLA-1 study in BRCA mutation-positive patients was more than 90% while the 2-year PFS was about 68% [21]. In the Ola arm of the SOLO1 study, the 1-year PFS was about 85%, but the 2-year PFS was also about 68% [16]. Thus, the PAOLA-1 study also seems to show the same phenomenon as described above in which the PFS of Bev-treated patients drops off after about 15 months (within 1 year after the start of maintenance therapy) and the effect of Bev is lost by 2 years. On the other hand, if we look at PDS R0 patients with BRCA mutations, the 2-year PFS of Ola + Bev is 96% [21], and the 2-year PFS of Ola is 80% [16], more than 1 year after the end of Bev treatment. Therefore, we do not see a drop in PFS after stopping Bev and a loss of Bev effect at 2 years. This is a direct comparison of data from different clinical trials and is also a comparison between subgroups, so it is not a scientifically valid data analysis. However, gynecological oncologists, who must continue to treat their current patients based on existing data, must keep this data in mind until new evidence becomes available. The 2-year PFS of 96% is impressive and suggests that a cure can be expected, and for gynecological oncologists, the current PDS R0 data from the PAOLA-1 study will motivate them to strive to achieve PDS R0.
Bev is essentially a cytostatic drug that postpones exacerbation events, but it does not have a cytotoxic effect and is not a drug that increases cure rates [2]. However, the results of PDS R0 in the PAOLA-1 study indicate that Bev may lead to enhancement of the cytotoxic effects of Ola. This enhancement may occur because Bev causes an increase in the penetration of Ola by preventing the formation of subclinical tumor masses. In addition, basic research has shown that hypoxia in tumors caused by angiogenesis inhibitors induces HRD [31, 32]. This finding might be the reason for the efficacy of Bev combined with Ola although in the PAOLA-1 study, Ola had no effect in HRp tumors, i.e. tumors with preserved DNA homologous recombination repair capacity. Even though the tumor mass has the characteristics of HRD, heterogeneity of “BRCAness” in individual tumor cells may exist, and hypoxia induced by Bev may have directed all tumor cells toward HRD, leading to enhancement of the effect of Ola. These ideas are only speculations based on the results of clinical trials and will be clarified by basic research in the future.
Comparison of Ola and Nira in BRCA mutation positive and high-risk group
For BRCA mutation positive high-risk cases other than PDS R0, the HR values for risk of progression were 0.34 for the SOLO1 study and 0.40 for the PRIMA study, so no clinically perceptible difference in the efficacy of Ola and Nira for BRCA mutated patients was indicated. At this point, there is one clear advantage of Ola over Nira, and that is its long term follow-up data, as it is a first-in-class product. Ola is generally terminated after 2 years, and many of the patients who have been relapse-free for 5 years may be cured. Data that indicate that the 5-year PFS was 42% in the high-risk group other than PDS R0 [16] would be attractive to physicians and patients. Therefore, in the absence of long-term follow-up data from the PRIMA study, Ola should be the first choice. In the future, Nira may be used instead of Ola when high expression of ABCB1 in the tumor is demonstrated.
When HGSC is diagnosed by cell block due to massive ascites or pleural effusion and PDS R0 is difficult due to old age or other reasons, it is not considered to be truly patient-centered medicine to perform a laparoscopic biopsy before chemotherapy. Even if tumor tissue is not collected prior to chemotherapy and chemotherapy is successful and tumor-based testing is no longer possible, germline mutations, which account for about 70% of BRCA mutations, can be examined by blood sampling, while somatic BRCA mutations, which only occur in tumor tissues and account for about 30% of BRCA mutations, cannot be determined. In this case, the number of patients receiving Ola would be slightly reduced because maintenance therapy with Ola alone is only approved for BRCA mutation cases in Japan. However, an option for the use of Nira that can be administered regardless of HRD status exists, and since no significant difference in the efficacy of Ola and Nira has been shown, a minimal disadvantage to patients by not performing laparoscopic biopsy exists. A slight possibility that innovative new treatment methods will be discovered in the future in which tissue biopsy prior to chemotherapy will lead to improved patient prognosis. However, at this point in time, laparoscopic biopsy in cases in which the histological type has been almost completely diagnosed by ascites sampling, while having research significance, is unlikely to lead to improved patient prognosis.
Comparison of Ola + Bev and Nira in HRD and high-risk group other than PDS R0
At this time, of the data from the PAOLA-1 study divided into the PDS R0 and high-risk groups, the data for HRD with BRCA wild type and GIS (GIS ≥ 42) are not available; only the data for HRD cases including BRCA mutation cases are available. It may be that the number of cases would be too small to evaluate if subgroup analyses were performed in great detail. In the HRD and high-risk cases, the Ola + Bev group in the PAOLA-1 study had 1- and 2-year PFS percentages of 86 and 56%, respectively, while the Nira group in the PRIMA study had 1- and 2-year PFS values of 70% and 48, respectively. Since the HR values were similar (0.39 for PAOLA-1 and 0.43 for PRIMA), Ola and Nira were probably equivalent in these cases. Some people often suggest that even if Ola and Nira are equivalent, Ola in the PAOLA-1 study is better than Nira in the PRIMA study because of the Bev base. However, considering that Bev ends within 1 year of the start of maintenance therapy and the benefit of Bev is lost soon thereafter, Ola + Bev in the PAOLA-1 study is not superior to Nira in the PRIMA study. Similar to results mentioned above, in a case in which HGSC was already diagnosed by ascites sampling and the treatment plan did not include PDS, laparoscopic biopsy used to obtain the option of Ola + Bev by myChoice® test despite the presence of Nira, is not considered for improving a patient’s prognosis.
Comparison between Bev and Nira in HRp tumors
The cases in the GOG218 study for Bev [33] are quite similar to the PRIMA study for Nira in that they are high risk cases other than PDS R0, and the Kaplan–Meier curve for GOG218 cases without HRR pathway gene mutations has been published [34]. However, as we reported, no correlation between mutations of HRR pathway genes other than BRCA1/2 and HRD score (GIS in myChoice®) was found [35]. The hazard ratio for PFS in the GOG218 study was obtained from the start of initial chemotherapy rather than from the start of maintenance therapy. Therefore, when comparing the data of the GOG218 cases without mutations in the HRR pathway genes with the data of the HRp cases in the PRIMA trial, one should keep in mind that several differences exist. A minimal difference in the Kaplan–Meier curve for PFS was found, which suggests a poor prognosis anyway.
An interim analysis of overall survival (OS) in the PRIMA study showed an HR of 0.51 in the HRp group, suggesting that Nira may extend OS [23]. However, the biggest concern with the PRIMA study data is the effect of individualized starting doses on HRp cases. Patients weighing less than 77 kg and/or those with platelet counts less than 150,000/μl are started at 300 mg/day for the fixed starting dose and 200 mg/day for the individualized starting dose. In the EMA Assessment Report, a detailed analysis of this group showed that for HRp, starting Nira at 300 mg/day prolonged the median PFS by 2.8 months with an HR of 0.61, while starting Nira at 200 mg/day prolonged median PFS by only 0.1 month with a HR of 0.75 (Table 2) [36]. For Nira maintenance therapy for platinum-sensitive recurrence in gBRCA wild-type patients, the NOVA study was performed at a fixed starting dose in the Western countries with a HR of 0.45 [38], and the NORA study was performed at an individualized starting dose in China with a HR of 0.40 [39], and the results from both studies were comparable. However, the gBRCA wild-type tumors treated with maintenance therapy for platinum-sensitive relapse are likely to include many tumors with HRD due to sBRCA mutations or other reasons, which is very different from the “HRp tumors that responded to TC on first-line therapy” in the PRIMA study. In addition, HRp tumors may reach the therapeutic range only after high doses of Nira are administered. Therefore, it cannot be denied that 300 mg/day for a specific time period may be important in the treatment of HRp tumors even though about 80% of patients will eventually receive a reduced dose of 200 mg/day or less. The EMA concluded that “For the HRp population, the 200 mg dose seems to be of lower efficacy compared to 300 mg”. However, EMA also stated that “The PRIMA study was not initially designed with the intent of studying different starting doses and considering that the lower dose has only been tested in limited number of subjects, it is realized that the study does not have the statistical power to allow any firm conclusions to be drawn in regards to the 200 mg starting dose compared to the 300 mg starting dose”. It is unfortunate that only the individualized starting dose is approved in the package insert, despite the fact that clinical trials were conducted in Japan with a fixed starting dose of 300 mg/day. In the PRIMA study, 200 mg/day was used in the last one-third of patients enrolled, so the follow-up period for those patients was short. It will be interesting to see whether the long-term follow-up data from the PRIMA trial and real-world data will show a clear antitumor effect of Nira at 200 mg/day for HRp tumors.
The enrollment criteria for the PRIMA study state that “Patients who have received bevacizumab with their first-line platinum-based therapy but are unable to receive bevacizumab as maintenance therapy due to adverse events or for any other reason are not excluded from study as long as the last dose of bevacizumab was received ≥28 days prior to signing the main informed consent form” [23]. In fact, seven patients (six in the actual drug group and one in the placebo group) who were using Bev for the first chemotherapy were included [36]. The package insert of Zejula® in Japan also states that Nira was administered after a withdrawal period of 28 days or more when Bev was used at the time of first treatment. From the aspect of Bev, no evidence of Bev's efficacy when used in combination with chemotherapy without Bev's maintenance therapy has been reported, and changing to a PARP inhibitor without Bev in maintenance therapy would be inappropriate because Bev in combination with chemotherapy would lose its rationale. However the effect of Bev in prolonging PFS disappears after 2 years [2], so from a patient-centered perspective, a decision to switch to Nira may be made in patients who started chemotherapy with Bev if chemotherapy is successful and OS is expected to be more than 2 years. On the other hand, in many cases of HRp, the response to chemotherapy may be inadequate, and in such cases, it may be better to switch to a regimen using Bev rather than sticking to Nira.
It is even more difficult to decide what to do about maintenance therapy for patients with HRp and PDS R0 or optimal surgery. These patients should be expected to be cured or to survive for a long time based on their surgical outcomes, and without maintenance therapy, they will soon relapse. Neither the PRIMA nor GOG218 trials enrolled patients in this group, and a subgroup analysis of the ICON7 trial showed that Bev did not prolong PFS in this group [37]. Thus, despite the lack of much data, both Nira and Bev are still covered by insurance, and we can only make predictions based on other data to decide which one to use. We do not use Bev in this group because Bev is not a drug to increase cure rates or to provide long-term survival [2]. As mentioned above, it is not known how effective Nira at 200 mg/day is for HRp tumors, but our policy is to use Nira for the cytotoxic effect of PARP inhibitors. Some concern about the use of Nira in patients with no residual tumor and no known response to chemotherapy exists; however, based on data showing that Nira was more effective in IDS R1 patients, we believe that the efficacy of Nira is not necessarily related to the response rate to chemotherapy.
In the JGOG3016 study, dose-dense TC, a regimen of increasing doses of paclitaxel, led to a prolongation of OS compared to TC in HGSC [40]. Basic research has shown that tumors with BRCA1 mutations are resistant to taxanes [41], and conversely, HRp tumors may be sensitive to taxanes. Therefore, HRp tumors may benefit from dose-dense TC when used as the initial chemotherapy regimen. In the VELIA study, which used veliparib in combination with chemotherapy plus maintenance, the chemotherapy regimen consisted of dose-dense TC and conventional TC in an almost 50:50 ratio. The risk of progression in the dose-dense TC group when compared with that in the TC group was HR 1.05 for patients with BRCA1/2 mutations, HR 0.77 for patients with wild-type BRCA1/2 and positive HRD by GIS, and HR 0.64 for patients with HRp, indicating a better prognosis in the dose-dense TC group [42]. The VELIA study was not a randomized controlled trial of TC versus dose-dense TC, so some bias in this comparison can be shown, but it seems to be a proof-of-concept based on the basic research data. Therefore, we use dose-dense TC as a chemotherapy regimen before Nira for HGSC patients with HRp who are younger and want to increase the intensity of treatment in expecting to achieve long-term survival. However, since dose-dense TC was not superior to TC in the ICON8 study [43], and there is no evidence yet on the efficacy of dose-dense TC followed by Nira maintenance regimen, whether our policy is really a good one or not needs further investigation.
About surgery; PDS or NAC/IDS?
Evidence for PDS R0
The current Japanese ovarian cancer treatment guidelines from recommend aiming for PDS R0, with an evidence level of A [44]. However, no randomized controlled trials that prove the benefit of PDS R0 have been published. The most famous paper cited in the guidelines is a meta-analysis of randomized controlled trials for chemotherapy, which reported that PDS R0 had a better prognosis than residual tumor (R1) in a subgroup analysis [45]. However, these data did not randomize resectable cancers into R0 and R1, and the R1 cases were likely to include those with truly unresectable lesions, so a bias about the disease state existed, and strictly speaking, no high level of evidence that striving for PDS R0 really improves prognosis has been found. However, a clinical trial that would allow a tumor to remain without removing it would be ethically difficult to execute, and it is unavoidable that a paper with a high level of evidence cannot be cited in the guidelines. If the reason for the lack of data is not “because it is not true” but “because it is ethically difficult to conduct a clinical trial”, the lack of data with a high level of evidence does not provide a reason not to aim for PDS R0. One of the papers cited in the guideline was published by the Mayo Clinic in the United States and states that surgical procedures have been standardized since 2007 to achieve a PDS R0 rate of more than 50% at stage IIIC. Even in the absence of PARP inhibitors, a PDS R0 at stage IIIC was associated with a 5-year PFS of about 40% [46], and gynecological oncologists, including Japanese, should aim for that surgical level.
Recently, a paper was published in which HGSCs were considered a mixture of chemotherapy-sensitive and -resistant cells and were simulated with a model in which chemotherapy in the presence of a tumor mass would increase the number of chemotherapy-resistant clones [47]. This study indicated that the difference in prognosis between PDS and NAC/IDS is significant when the surgical residual tumor is less than 1 mm, and that complete resection of PDS is important for improving survival. This study demonstrates the benefit of surgery in a manner other than a clinical trial and raises awareness of the importance of avoiding chemotherapy for grossly visible tumor masses.
Complete resection, including invisible lesions
Ninety-seven percent of ovarian cancers express folate receptors. In surgery for advanced ovarian cancer, intravenous infusion of pafolacianine (CYTALUX®), a folic acid conjugated with an indocyanine green (ICG)-like fluorescent substance, was shown to be taken up by ovarian cancer cells expressing folate receptors, and the tumor can be depicted in green intraoperatively by an imaging system using near-infrared light. In a phase III clinical trial, pafolacianine was shown to be useful for detecting additional lesions in 27% of 134 patients even though the lesions were mainly R0 [48]. Based on the results of this clinical trial, pafolacianine (CYTALUX®) was FDA-approved for surgery for advanced ovarian cancer in November 2021. Thus, in the future treatment of ovarian cancer, complete tumor resection is required, including not only gross R0 but also invisible tumors.
What are cases in which PDS is difficult?
If PDS is difficult, NAC + IDS should be performed, but it is controversial in which cases PDS should be considered difficult. It should be noted that the evidence for diagnostic laparoscopy is for predicting whether complete resection or optimal surgery is possible, and no evidence for diagnostic laparoscopy only for observation and pathological diagnosis by biopsy, which is often done in Japan, exists. Recently, a diagnostic laparoscopic predictive index has been proposed to predict whether or not optimal surgery is possible [49], and this index is also cited in the Japanese ovarian cancer treatment guidelines [44]. A predictive index of 0 to 6 indicates that PDS is relatively easy, 8 to 12 indicates that PDS is difficult, and 14 indicates that PDS is impossible. For example, extensive invasion covering most of the diaphragmatic surface plus invasion requiring resection of the gastrointestinal tract plus involvement of the liver surface would result in a predictive index of 6, which would be considered “relatively easy” although this surgery does not seem to be easy in majority of institutions in Japan. No evidence that NAC/IDS can be performed instead of PDS in patients with a predictive index of 6 or lower has been reported. In patients with a predictive index of 8 to 12, NAC/IDS was reported to be better than PDS because it did not lead to worse prognosis and caused a reduction in complications [50]. However, it should be noted that these results are data from the era before the advent of PARP inhibitors.
Of course, the risk of complications from PDS should be considered in patients who are older than 80 years old or who have poor performance status. The Mayo Clinic has reported a simple triage algorithm for assessing the risk of complications and deciding whether or not to perform PDS (Fig. 3) [51]. Older patients often do not want to undergo surgery when the risks of surgery for advanced ovarian cancer are carefully explained to them. Among the criteria in this algorithm, albumin < 3.5 g/dL applies to many patients with pleural effusion or ascites, and if these patients undergo NAC, the ratio of PDS will be too low. At our hospital, we do not place much importance on this criterion and perform PDS even if the albumin level is < 3.5 g/dL if we judge that PDS is possible.
Mayo triage algorithm [51]. In our hospital, we do not use the criteria for serum albumin levels
Surgical policy for ovarian cancer in our hospital
After the results of the PDS R0 subgroup of PAOLA-1 were reported at IGCS in September 2020, our hospital changed its policy to aggressively aim for PDS R0 the following month. We try to perform surgery within three weeks of the initial diagnosis of advanced ovarian cancer. In our hospital, PDS of advanced ovarian cancer is rarely completed only by gynecological oncologists, and we usually request support from surgery, urology, and post-operative intensive care unit (ICU) management. If symptoms due to ascites accumulation are severe during the pre-operative waiting period, cell-free and concentrated ascites reinfusion therapy (CART) can be used to extend the waiting period. However, in the pre-operative period of advanced ovarian cancer, a certain number of surgeries will inevitably be cancelled because a patient’s general condition can change from moment to moment. If we are not confident that PDS R0 is possible based on the pre-operative imaging examination, we observe the inside of the abdominal cavity by laparoscopy, and on the same day, we can switch to PDS by laparotomy. If the facility allows laparoscopic observation within a week after the initial diagnosis and then PDS on another day, the frequency of PDS cancellations may be reduced. The predictive index [49] is used as a reference during laparoscopic observation, but it is not easy to objectively judge whether PDS R0 is possible. In principle, all disseminated lesions in the diaphragm can be removed, and lesions that can be removed by bowel resection or stoma construction can be removed. We consider PDS R0 to be infeasible in patients with disseminated lesions that have spread to the entire serosa of the small intestine or in patients with disseminated peritoneal lesions that are diffusely thickened throughout the peritoneum without forming a mass. Rather than scoring in detail, we try to aim for PDS R0 as much as possible, being aware that whether or not to perform PDS R0 is directly related to the life or death of the patient. As a result, in the last year, PDS R0 was achieved in about half of the HGSC stage III-IV casesrs under the age of 75.
Ovarian cancer treatment in Japan in the future
In the past, when PARP inhibitors were not available, advanced ovarian cancer was a disease in which even after achieving PDS R0 and chemotherapy, many patients would relapse, undergo repeated chemotherapy, and eventually be transferred to palliative care. Even today, a significant number of ovarian cancer cases are practically impossible to cure due to old age and other reasons. The strategy of NAC and reduction surgery to avoid colostomy was a promising option rather than having a colostomy to achieve PDS R0. For example, in Japan, there are many facilities that do not perform complete resection by PDS when multiple parts of the large intestine need to be resected, because the length of the large intestine is not sufficient for anastomosis with the rectum or the frequency of suture failure increases.
However, with the advent of PARP inhibitors, the treatment of at least half of advanced ovarian cancer cases has changed dramatically, and the goal of treatment is shifting from “maintaining QOL as a chronic disease for a long time” to “cure”. In particular, the 2-year PFS of >90% for Ola + Bev in patients with PDS R0 and HRD is exactly at the level for which we have been striving. This is a subgroup analysis, and the level of evidence is low, but the level of evidence is usually lower for surgical data than for drug data. Although the situation varies by hospital, country, and patient condition, the importance of achieving PDS R0 has increased compared to the past.
In Japan, facilities that perform surgery for gynecological cancers are not centralized and there are many small to medium-sized facilities that treat ovarian cancer. The number of cases at our hospital (Kindai University Hospital) is average among Japanese university hospitals, and frequency of PDS for advanced ovarian cancer is about one case per month. There are few facilities in Japan that can participate in the TRUST study conducted by the AGO study group, which requires more than 36 debulking surgeries per year [52]. Centralization of gynecological cancer facilities is a challenge for Japan.
This article discusses the first-line treatment of ovarian cancer based on the evidence available as of December 2021. Evidence is constantly being added, so there is a need to revise this content in the future.
References
WHO Solidarity Trial Consortium (2021) Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med 384:497–511
Nakai H, Matsumura N (2022) The roles and limitations of bevacizumab in the treatment of ovarian cancer. Int J Clin Oncol, paper in press
Li H, Liu ZY, Wu N et al (2020) PARP inhibitor resistance: the underlying mechanisms and clinical implications. Mol Cancer 19:107
Murai J, Huang SN, Das BB et al (2012) Trapping of PARP1 and PARP2 by Clinical PARP inhibitors. Cancer Res 72:5588–5599
Hopkins TA, Shi Y, Rodriguez LE et al (2015) Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol Cancer Res 13:1465–1477
Zandarashvili L, Langelier MF, Velagapudi UK et al (2020) Structural basis for allosteric PARP-1 retention on DNA breaks. Science 368:eaax6367
PMDA. Lynparza® drug interview form. 2021; 8th edition. https://www.pmda.go.jp/
PMDA. Zejula® drug interview form. 2021; 3rd edition. https://www.pmda.go.jp/
Sun K, Mikule K, Wang Z et al (2018) A comparative pharmacokinetic study of PARP inhibitors demonstrates favorable properties for niraparib efficacy in preclinical tumor models. Oncotarget 9:37080–37096
Patch AM, Christie EL, Etemadmoghadam D et al (2015) Whole-genome characterization of chemoresistant ovarian cancer. Nature 521:489–494
Borst P, Schinkel AH (2013) P-glycoprotein ABCB1: a major player in drug handling by mammals. J Clin Invest 123:4131–4133
Vaidyanathan A, Sawers L, Gannon AL et al (2016) ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br J Cancer 115:431–441
Gray S, Khor XY, Yiannakis D (2019) Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Rep 12:e230738
Wang Q, Zhang F, Gao H et al (2021) Successful treatment of a patient with brain metastases from endometrial cancer using Niraparib: a case report. Ann Palliat Med 10:818–827
Del Campo JM, Matulonis UA, Malander S et al (2019) Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA Trial. J Clin Oncol 37:2968–2973
Banerjee S, Moore KN, Colombo N et al (2021) Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 22:1721–1731
DiSilvestro P, Colombo N, Scambia G et al (2020) Efficacy of maintenance olaparib for patients with newly diagnosed advanced ovarian cancer with a BRCA mutation: subgroup analysis findings from the SOLO1 Trial. J Clin Oncol 38:3528–3537
Ray-Coquard I, Pautier P, Pignata S et al (2019) Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416–2428
Takaya H, Nakai H, Sakai K et al (2020) Intratumor heterogeneity and homologous recombination deficiency of high-grade serous ovarian cancer are associated with prognosis and molecular subtype and change in treatment course. Gynecol Oncol 156:415–422
Lorusso D, Lotz JP, Harter P et al (2020) Maintenance olaparib plus bevacizumab after platinum-based chemotherapy plus bev in patients with newly diagnosed advanced high-grade ovarian cancer: Efficacy by BRCA1 or BRCA2 mutation in the phase III PAOLA-1 trial. J Clin Oncol 38(Suppl 15):6039
Harter P, Petran D, Scambia G et al (2020) Efficacy of maintenance olaparib plus bevacizumab by biomarker status in clinical higher- and lower-risk patients with newly diagnosed, advanced ovarian cancer in the PAOLA-1 trial. Int J Gynecol Cancer 30(Suppl 3):A13–A14
González-Martín A, Tazi Y, Heitz F et al (2020) Maintenance olaparib plus bevacizumab in patients with newly diagnosed advanced high-grade ovarian carcinoma: final analysis of second progression-free survival in the phase III PAOLA-1/ENGOT-ov25 trial. Ann Oncol 31(Suppl 4):S1163–S1164
González-Martín A, Pothuri B, Vergote I et al (2019) Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391–2402
O’Cearbhaill R, Pérez-Fidalgo JA, Monk B et al (2021) Efficacy of niraparib by timing of surgery and residual disease: a post-hoc analysis of patients in the PRIMA/ENGOT-OV26/GOG-3012 study. Gynecol Oncol 162(Suppl 1):S65
González-Martín A, Matulonis U, Korach J et al (2021) Niraparib efficacy and safety in patients with BRCA mutated ovarian cancer: Results from three phase 3 niraparib trials. J Clin Oncol 39(Suppl 15):5518
Coleman RL, Fleming GF, Brady MF et al (2019) Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 381:2403–2415
Zejula® Guide to Appropriate Use (2021) https://www.takedamed.com/medicine/zejula/pdf/common/1-5-5462.pdf
Mirza MR, González-Martín A, Graybill W et al (2020) Evaluation of an individualized starting-dose of niraparib in the PRIMA/ENGOT-OV26/GOG-3012 study. J Clin Oncol 38(Suppl 15):6050
Takehara K, Matsumoto T, Hamanishi J et al (2021) Phase 2 single-arm study on the safety of maintenance niraparib in Japanese patients with platinum-sensitive relapsed ovarian cancer. J Gynecol Oncol 32:e21
Okamoto A, Kondo E, Nakamura T et al (2021) Phase 2 single-arm study on the efficacy and safety of niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer. J Gynecol Oncol 32:e16
Chan N, Pires IM, Bencokova Z et al (2010) Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res 70:8045–8054
Kaplan AR, Gueble SE, Liu Y et al (2019) Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci Transl Med 11:eaav4508
Burger RA, Brady MF, Bookman MA et al (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473–2783
Norquist BM, Brady MF, Harrell MI et al (2018) Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: an NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 24:777–783
Takaya H, Nakai H, Takamatsu S et al (2020) Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci Rep 10:2757
European Medicines Agency. Zejula Assessment report. EMA/531223/2020
Perren TJ, Swart AM, Pfisterer J et al (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484–2496
Mirza MR, Monk BJ, Herrstedt J et al (2016) Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154–2164
Wu XH, Zhu JQ, Yin RT et al (2021) Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol 32:512–521
Katsumata N, Yasuda M, Isonishi S et al (2013) Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14:1020–1026
Sung M, Giannakakou P (2014) BRCA1 regulates microtubule dynamics and taxane-induced apoptotic cell signaling. Oncogene 33:1418–1428
Okamoto A, Fleming G, Bookman M et al (2020) Veliparib with carboplatin and paclitaxel in frontline high-grade serous ovarian cancer: Efficacy and safety of paclitaxel weekly and every 3 weeks in the VELIA study. Ann Oncol 31(Suppl 4):S618
Clamp AR, James EC, McNeish IA et al (2019) Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 394:2084–2095
Tokunaga H, Mikami M, Nagase S et al (2021) The 2020 Japan Society of Gynecologic Oncology guidelines for the treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. J Gynecol Oncol 32:e49
du Bois A, Reuss A, Pujade-Lauraine E et al (2009) Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115:1234–1244
Wallace S, Kumar A, Mc Gree M et al (2017) Efforts at maximal cytoreduction improve survival in ovarian cancer patients, even when complete gross resection is not feasible. Gynecol Oncol 145:21–26
Gu S, Lheureux S, Sayad A et al (2021) Computational modeling of ovarian cancer dynamics suggests optimal strategies for therapy and screening. Proc Natl Acad Sci USA 118:e2026663118
Tanyi JL, Chon HS, Morgan MA et al (2021) Phase 3, randomized, single-dose, open-label study to investigate the safety and efficacy of pafolacianine sodium injection (OTL38) for intraoperative imaging of folate receptor positive ovarian cancer. J Clin Oncol 39(Suppl 15):5503
Fagotti A, Vizzielli G, Iaco PD et al (2013) A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am J Obstet Gynecol 209:462.e1-462.e11
Fagotti A, Ferrandina MG, Vizzielli G et al (2020) Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int J Gynecol Cancer 30:1657–1664
Narasimhulu DM, Kumar A, Weaver AL et al (2019) Using an evidence-based triage algorithm to reduce 90-day mortality after primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol 155:58–62
Reuss A, du Bois A, Harter P et al (2019) TRUST: Trial of Radical Upfront Surgical Therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int J Gynecol Cancer 29:1327–1331
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hidekatsu Nakai declare no conflict of interest regarding with this article. Noriomi Matsumura received lecture fee from Chugai Pharmaceutical, AstraZeneca, and Takeda Pharmaceutical. Noriomi Matsumura received research grant from AstraZeneca. Noriomi Matsumura is also an outside director of Takara Bio.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is translated and modified from a Japanese review article to be published in the Journal (ACTA OBSTETRICA ET GYNAECOLOGICA JAPONICA) of the Japan Society of Obstetrics and Gynecology.
About this article
Cite this article
Nakai, H., Matsumura, N. Individualization in the first-line treatment of advanced ovarian cancer based on the mechanism of action of molecularly targeted drugs. Int J Clin Oncol 27, 1001–1012 (2022). https://doi.org/10.1007/s10147-022-02163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02163-3