Abstract
Background
Although FOLFIRINOX is currently one of the standard therapies for chemotherapy-naïve patients with metastatic pancreatic cancer (MPC), the high rate of febrile neutropenia (FN) presents a clinical problem. This study aimed to evaluate the safety and efficacy of primary prophylactic pegfilgrastim with FOLFIRINOX in Japanese MPC patients.
Methods
FOLFIRINOX (intravenous oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, levofolinate 200 mg/m2, 5-fluorouracil (5-FU) bolus 400 mg/m2 and 5-FU 46 h infusion 2400 mg/m2) and pegfilgrastim 3.6 mg on day 4 or 5, every 2 weeks was administered to previously untreated MPC patients. The primary endpoint was the incidence of FN during the first 3 cycles. The planned sample size was 35 patients, but the trial was predefined to discontinue enrollment for safety if 4 patients developed FN.
Results
At the enrollment of 22 patients, 4 patients developed FN in the first cycle, resulting in an incidence of FN of 18% {95% confidence interval [CI], 0.5–40.3%}, and enrollment was discontinued early. The incidence of grade 3 or higher neutropenia was 36.4%. Median relative dose intensities during the initial 3 cycles of oxaliplatin, irinotecan, bolus 5-FU, infusional 5-FU, and levofolinate maintained high (100%, 89.0%, 100%, 66.0%, and 100%, respectively). Response rate and median overall survival were 54.5% (95% CI 32.7–74.9) and 15.7 months (95% CI 7.9–18.8), respectively.
Conclusions
This phase II study could not demonstrate any reduction in the incidence of FN, nevertheless some patients experience benefits for efficacy by maintaining dose intensity using prophylactic pegfilgrastim.
Trial registration
http://www.umin.ac.jp/ctr/index-j.htm, UMIN000017538. Date of registration: May/13/2015
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer (PC) is a highly lethal cancer with a 5-year survival rate of 2–3%, and is the fourth leading cause of cancer-related mortality in Japan [1]. As most patients are initially diagnosed with PC at an unresectable stage, chemotherapy plays the most important role in the treatment of advanced PC at presentation.
FOLFIRINOX [oxaliplatin, irinotecan, 5-fluorouracil (5-FU), and leucovorin] showed a significant improvement in overall survival (OS) compared to gemcitabine (GEM) for metastatic pancreatic cancer (MPC) in the ACCORD11/PRODIGE4 trial [2]. FOLFIRINOX has become the standard treatment for MPC. In Japan, a phase II trial (the LOHP-PII-05 trial) was conducted to evaluate the efficacy and safety of FOLFIRINOX in MPC patients [3]. This efficacy was similar to that reported in the ACCORD11/PRODIGE4 trial, but incidences of grade 3 or 4 toxicities, particularly neutropenia and febrile neutropenia (FN), were higher in the LOHP-PII-05 trial (77.8% and 22.2%, respectively) than in the ACCORD11/PRODIGE4 trial (45.5% and 5.4%, respectively). FN is a serious, potentially life-threatening toxicity and severe neutropenia and FN are reportedly observed more frequently in Asian populations, including Japanese, than in non-Asian populations [3,4,5]. Hence, there is a clinical need to reduce the incidence of FN in Japanese patients with MPC treated using FOLFIRINOX.
Granulocyte colony-stimulating growth factor (G-CSF) decreases the risk of FN in patients receiving myelosuppressive chemotherapies [6, 7]. According to several clinical oncology practice guidelines, prophylactic G-CSF is recommended when the risk of FN exceeds 20% [8,9,10]. Pegfilgrastim, a pegylated form of filgrastim, has a long half-life. A phase III placebo-controlled, double-blinded, randomized trial of pegfilgrastim in patients with breast cancer who received docetaxel in Europe and North America demonstrated significantly reduced incidences of FN, FN-related hospitalization, and antibiotic use [11]. Pegfilgrastim has, therefore, been approved in many countries. A recent systematic review of randomized clinical trials designed to investigate the impact of G-CSF on mortality and FN has revealed that prophylactic use of G-CSF reduces the rates of FN, dose reduction and treatment delay [12].
Prophylactic pegfilgrastim was expected to reduce the incidence of FN and allow maintenance of the dose intensity of FOLFIRINOX, and finally maximize the efficacy of FOLFIRINOX. We, therefore, conducted a phase II trial to evaluate the safety and efficacy of primary prophylactic pegfilgrastim in Japanese patients with MPC who received FOLFIRINOX.
Patients and methods
Study design
This open-label, single-arm phase II trial was conducted at 4 institutions. This clinical trial was conducted with the approval of the review boards of each participating institution and in accordance with the Declaration of Helsinki. This trial is registered with UMIN-CTR (http://www.umin.ac.jp/ctr/index-j.htm; identification number UMIN000017538).
Patients
Inclusion criteria were as follows: histologically or cytologically confirmed pancreatic adenocarcinoma or adenosquamous carcinoma; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1; age 20–75 years; MPC with at least one measurable lesion; and adequate hematological, liver, and renal functions (hemoglobin ≥ 9.0 g/dL, white blood cell count ≤ 10,000/mm3, neutrophil count ≥ 2000/mm3, platelet count ≥ 100,000/mm3, total bilirubin ≤ upper limit of normal; aspartate transaminase and alanine transaminase ≤ 2.5 × upper limit of normal; and creatinine ≤ 1.2 mg/dL).
Patients were excluded if they had: received prior chemotherapy or radiation therapy; grade 2 or higher peripheral sensory neuropathy; blood transfusion, blood products, or hematopoietic growth factor preparations such as G-CSF within 7 days before enrollment; uridine diphosphate glucuronosyltransferase (UGT) genetic polymorphisms of homozygous UGT1A1*28 or UGT1A1*6 or heterozygous UGT1A1*6 and UGT1A1*28; apparent coelomic fluid (pleural effusion, ascites, or pericardial fluid) or peritoneal dissemination; diarrhea including watery stools within 3 days before enrollment; poorly controlled diabetes; synchronous or metachronous double cancer, excluding carcinoma in situ or intramucosal carcinoma cured by local treatment; active infection; or other serious concomitant diseases. All the above eligibility criteria were set in the same as the LOHP-PII-05 trial.
Treatment
FOLFIRINOX was given every 2 weeks, as follows: oxaliplatin 85 mg/m2 infused over 120 min, immediately followed by levofolinate 200 mg/m2 infused over 120 min with the addition, after 30 min, of irinotecan 180 mg/m2 infused over 90 min, followed by 5-FU 400 mg/m2 bolus, followed by 2400 mg/m2 continuous infusion for 46 h. Subcutaneous injection of pegfilgrastim 3.6 mg was given on day 4 or day 5. Use of pegfilgrastim was mandated in the first 3 cycles, then each investigator chose whether to use pegfilgrastim in subsequent cycles.
A 5-HT3 receptor antagonist and dexamethasone were administered prior to FOLFIRINOX. Selective neurokinin 1 receptor antagonistic antiemetics were recommended to alleviate nausea and vomiting. Treatment was continued until disease progression, unacceptable toxicity, discontinuation as decided by the investigator, or patient refusal.
Chemotherapy was delayed until recovery from the following criteria: neutrophil count < 1500/mm3; platelet count < 75,000/mm3; total bilirubin > 1.5 mg/dL; grade 3 or higher peripheral sensory neuropathy; grade 2 or higher diarrhea; and watery stools. When a predefined toxic event in the protocol occurred, dose adjustment was required. The reduced dose was set at 150 mg/m2 and 120 mg/m2 for irinotecan, 65 mg/m2 and 50 mg/m2 for oxaliplatin, and 1800 mg/m2 and 1200 mg/m2 for infusional 5-FU.
Outcomes and assessments
The primary endpoint was the incidence of FN during the first 3 cycles of chemotherapy. FN was defined in this study as a single body temperature measurement > 38.3 °C, or a temperature ≥ 38.0 °C sustained over 1 h, with a neutrophil count < 1000/mm3 within 1 day of the identified fever. According to the LOHP-PII-05 study [3], all patients who developed FN developed the adverse reaction during the first cycle of therapy, and more than half of the patients required dose reduction of either oxaliplatin or irinotecan due to the development of adverse events during the first 3 cycles of treatment. Hence, the evaluation period was set to be during the first 3 cycles of therapy.
Secondary endpoints were OS, progression-free survival (PFS), response rate (RR), and relative dose intensity (RDI) during the first 3 cycles of chemotherapy, and safety for all patients. OS was defined as the time from study enrollment to death from any cause. PFS was defined as the time from study enrollment to disease progression or death. RDI was defined as the proportion of delivered dose intensity to the planned dose intensity for each agent from first day of first cycle to last day of third cycle. Patients were evaluated for toxicities during the entire course. Complete blood counts, blood chemical tests, and physical examinations were carried out at least every 2 weeks. Computed tomography was performed at least every 6 weeks. RR was assessed based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 guidelines. Toxicities were graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
Incidence of FN during the first 3 cycles of pegfilgrastim with FOLFIRINOX was expected to be lower than that from the LOHP-PII-05 trial (22.2%). A minimum sample size of 32 was required for a one‐sided α of 0.1 and β of 0.2, with an expected FN incidence of 5% and a threshold incidence of 20% using the binomial test. The target sample size was set at 35 patients, considering that ineligible patients would be enrolled in this trial. If more than 4 of the 35 patients were to develop FN, the upper limit of the 95% confidence interval (CI) for FN incidence would be > 20%. As a result, enrollment was to be stopped immediately for the safety of patients as soon as FN was identified in 4 patients. The Kaplan–Meier method was used to estimate survival curves. All statistical analyses were performed with EZR ver.1.51 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Between May 2015 and July 2016, a total of 22 patients were enrolled from the 4 institutions. FN was identified in a fourth patient after enrollment of 22 patients, and further enrollment was, therefore, suspended.
Patient characteristics at baseline are shown in Table 1. Median age was 61 years (45–73 years), and 63.6% of patients had PS 0. The primary site of the tumor was the head of the pancreas in 68.2% of patients and the most common site of metastasis was the liver. A biliary stent was present in 22.3% of patients and 54.5% of patients showed a heterozygous UGT1A1 genotype.
Treatment exposure
All patients received the study drugs, and 20 patients completed 3 cycles of treatment. The median number of treatment cycles was 9 (1–41). Median relative dose intensities during the first 3 cycles of oxaliplatin, irinotecan, bolus 5-FU, infusional 5-FU, and levofolinate were 100%, 89.0%, 66.0%, 100%, and 100%, respectively. Dose reductions and/or treatment delays occurred in 14 patients (63.6%) during the first 3 cycles. The most common reasons for treatment delay and/or dose reductions were FN (4 patients), diarrhea (3 patients), and investigator decision (serum C-reactive protein elevation, 2 patients; serum aminotransferase elevation, 2 patients). The major reasons for treatment discontinuation were disease progression (14 patients, 63.6%) and adverse events (5 patients, 22.7%: biliary tract infection, 2 patients; FN, 1 patient; catheter-related infection, 1 patient; diarrhea, 1 patient).
Safety
Four patients developed FN during the first 3 cycles (18.2%; 95% CI 0.5–40.3%). Detailed characteristics of these four patients are shown in Table 2. All instances of FN developed during the first cycle, but no other specific clinical features were observed in these patients.
Treatment-related adverse reactions occurring in all 22 patients are summarized in Table 3. Treatment-related serious adverse events occurred in 5 patients (22.7%), comprising FN in 4 patients and grade 3 diarrhea in 1 patient. Grade 3–4 toxicities occurred in 12 patients (54.5%). The major grade 3–4 hematological toxicities were neutropenia (36.4%), leucopenia (36.4%), and thrombocytopenia (22.7%), and the major grade 3–4 non-hematological toxicities were anorexia (13.6%), diarrhea (4.5%), nausea (4.5%), and peripheral neuropathy (13.6%). Bone pain is the common adverse event of interest for pegfilgrastim, but occurred in only 1 patient (4.5%) in this study. No treatment-related deaths were encountered.
Efficacy
Response was classified as partial response in 12 patients, stable disease in 8 patients, and progressive disease in 2 patients. The overall RR and disease control rate in all patients were 54.5% (95% CI 32.7–74.9%) and 90.1% (95% CI 69.4–98.4%), respectively. One patient achieved complete response of peritoneal metastases under this regimen, and underwent distal pancreatectomy and splenectomy for the primary tumor. In this case, pathological findings revealed R0 and he remained alive without recurrence for 2 years.
At the time of analysis, 19 patients had died, 3 patients were alive, and no patients had been lost to follow-up. Median OS was 15.7 months (95% CI 7.9–18.8 months) (Fig. 1), and median PFS was 6.4 months (95% CI 4.0–13.0 months) (Fig. 2).
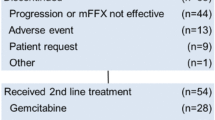
Of the 22 enrolled patients, 19 received second-line therapy. The most common main treatments were GEM + nab-paclitaxel (11 patients) and GEM alone (2 patients).
Discussion
To the best of our knowledge, this represents the first prospective study to evaluate the safety and efficacy of primary prophylactic pegfilgrastim in Japanese MPC patients who received FOLFIRINOX. Current clinical practice guidelines [8,9,10] recommend primary prophylactic G-CSF when the risk of FN is > 20% in chemotherapy. An incidence of FN > 20% was reported in the LOHP-PII-05 trial [3], so the present study was conducted in expectation of a reduced incidence of FN with FOLFIRINOX for Japanese MPC patients. However, FN developed in 4 of the 22 patients (18.0%) during the first 3 cycles regardless of primary prophylaxis with pegfilgrastim. Our study thus failed to demonstrate a reduction in the incidence of FN by adding pegfilgrastim to FOLFIRINOX.
Patient characteristics also represent important risk factors for FN. Various retrospective studies have reported female sex, body mass index ≥ 25 kg/m2, biliary stent insertion, low pretreatment platelet count and UGT1A1 genetic polymorphisms as risk factors for FN in Asian PC patients treated with FOLFIRINOX [13, 14]. In addition, a proportion of patients with other malignancies still develop FN despite receiving prophylactic G-CSF. Lee et al. reported low pretreatment platelet count (< 15 × 104/μL) as a risk factor for FN among cancer patients with pegfilgrastim prophylaxis [15]. However, the 4 patients who developed FN in this study showed none of these features. We thus could not identify any specific cause for the failure to reduce the incidence of FN in the features of these patients.
Combination use of prophylactic pegfilgrastim with chemotherapy could contribute to reducing the frequency of grade 3–4 neutropenia and maintaining RDI [10]. Neutropenia was the most frequent cause for reducing RDIs of FOLFIRINOX in the LOHP-PII-05 trial. The frequency of grade 3 or 4 neutropenia in this study (36.4%) was lower than that in the LOHP-PII-05 trial (77.8%). Thus, the major reason for reducing RDIs was not neutropenia but was non-hematological toxicity in this study. In fact, RDIs during 3 cycles in this study (oxaliplatin 100%, irinotecan 89.0%, bolus 5-FU 66.0%, infusional 5-FU 100%, l-leucovorin 100%) were maintained high.
With regard to non-hematological toxicities, pegfilgrastim appeared well tolerated. The common adverse events associated with pegfilgrastim were bone pain (4.5%) and ALP elevation (86.4%), and no patients showed grade 3 or higher adverse events related to pegfilgrastim. The incidences of grade 3 or higher anorexia, nausea, diarrhea, and peripheral sensory neuropathy in this study resembled those in the LOHP-PII-05 trial (13.6%, 4.5%, 4.5% and 13.6% vs 11.1%, 8.3%, 8.3% and 5.6%, respectively). These results suggest that pegfilgrastim use did not increase the risk of non-hematological toxicities.
In our study, RR, DCR, PFS, and OS were 54.5%, 90.1%, 6.4 months, and 15.7 months, respectively. These results were better than those of both the ACCORD11/PRODIGE4 trial (31.6%, 70.2%, 6.4 months and 11.1 months, respectively) and the LOHP-PII-05 trial (38.9%, 69.4%, 5.6 months, and 10.7 months, respectively). In some types of cancer such as lung, breast, and ovarian cancers, maintaining RDI has been shown to improve clinical outcomes [16,17,18,19]. Regarding FOLFIRINOX for advanced PC, a few retrospective studies have reported the effect of RDI on efficacy. Lee et al. reported that a cumulative RDI of FOLFIRINOX > 70% was related to radiological response [20] and Kobayashi et al. reported that a high RDI (> 75%) for irinotecan within the first 2 cycles correlated positively with objective response [21]. The favorable RR in our study may have been obtained by maintaining RDI with primary prophylactic pegfilgrastim. On the other hand, while the most common second-line treatment in the ACCORD11/PRODIGE4 and LOHP-PII-05 trials was GEM alone, in our study, many patients received gem + nab-paclitaxel as second-line treatment. Thus, the favorable survival may have been influenced by the content of the second-line treatments.
Recently, some prospective and retrospective studies were conducted to evaluate the efficacy and safety profile of modified FOLFIRINOX therapy in patients with advanced PC. Ozaka et al. reported that the modified FOLFIRINOX regimen, in which bolus 5-FU is omitted and the dose of irinotecan is reduced (to 150 mg), without prophylactic pegfilgrastim administration, shows an improved safety profile with maintained efficacy in Japanese patients with MPC [22]. Based on this report, this modified FOLFIRINOX regimen has been used as the domestic standard in Japan. The modified FOLFIRINOX regimen including prophylactic pegfilgrastim administration has been reported from western countries to yield favorable results [23, 24]. With respect to adverse events other than FN, the incidence of grade 3 or higher thrombocytopenia in our study was higher than that in the Japanese modified FOLFIRINOX study (22.7 vs. 2.9%). Kajiyama et al. reported from a large national database, that pegfilgrastim increased the risk of thrombocytopenia in Japanese patients treated with antineoplastic agents [25]. Therefore, as not only the doses of FOLFIRINOX, but also the dose of pegfilgrastim could have had an influence on the incidence of severe thrombocytopenia, careful attention should be paid when adding pegfilgrastim administration to the modified FOLFIRINOX regimen for Japanese patients with PC.
The major limitation of the present study was that we used pegfilgrastim at a dose of 3.6 mg. According to a trial that verified the dose response of pegfilgrastim in Japanese patients, efficacy peaked at 3.6 mg [26]. The fixed dose of prophylactic pegfilgrastim in Japanese patients is 3.6 mg, as the dose approved by the Japanese healthcare system. On the other hand, pegfilgrastim is usually used at a dose of 6.0 mg in other countries, and Faris et al. reported that prophylactic pegfilgrastim at 6.0 mg with FOLFIRINOX for PC reduced the incidence of FN [27]. Hence, while pegfilgrastim 3.6 mg failed to reduce FN with FOLFIRINOX in Japanese MPC according to the present results, whether pegfilgrastim 6.0 mg could reduce FN remains unclear.
In conclusion, this phase II study was unable to demonstrate a reduction in the incidence of FN using primary prophylactic pegfilgrastim with FOLFIRINOX in Japanese MPC. However, some patients benefited from maintaining chemotherapy dose intensity by prophylactic pegfilgrastim. The combination of prophylactic pegfilgrastim may contribute to reducing the rate of neutropenia and maintaining RDI, and could lead to favorable antitumor effects. Further studies are warranted to determine the optimal use of primary prophylactic pegfilgrastim with FOLFIRINOX for Japanese PC patients.
References
Vital Statistics Japan (Ministry of Health, Labour and Welfare) [Center for Cancer Control and Information Services, National Cancer Center, Japan Web site]. Available at: https://ganjoho.jp/reg_stat/statistics/dl/index.html. Accessed Jan 2020
Conroy T, Desseigne F, Ychou MO et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Okusaka T, Ikeda M, Fukutomi A et al (2014) Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci 105:1321–1326
Hasegawa Y, Kawaguchi T, Kubo A et al (2011) Ethnic difference in hematological toxicity in patients with non-small cell lung cancer treated with chemotherapy: a pooled analysis on Asian versus non-Asian in phase II and III clinical trials. J Thorac Oncol 6:1881–1888
Gandara DR, Kasaguchi T, Crowlet J et al (2009) Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol 27:3540–3546
Crawford J, Ozer H, Stoller R et al (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325:164–170
Vogel CL, Wojtukiewicz MZ, Carroll RR et al (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer. A multicenter, double-blind, placebo controlled phase III study. J Clin Oncol 23:1178–1184
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199–3212
National Comprehensive Cancer Network. NCCN® Clinical practice guidelines in oncology: myeloid growth factors, version 2.2020. National Comprehensive Cancer Network. Available at: https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed Jan 2020
Green MD, Koelbl H, Baselga J et al (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35
Lyman GH, Dale DC, Culakova E et al (2013) The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 24(10):2475–2484
Keum J, Lee HS, Kang H et al (2020) Single-center risk factor analysis for FOLFIRINOX associated febrile neutropenia in patients with pancreatic cancer. Cancer Chemother Pharmacol 85(4):651–659
Sasaki M, Ueno H, Kuchiba A et al (2015) Risk factors for febrile neutropenia in patients with unresectable pancreatic cancer receiving FOLFIRINOX as the first-line treatment. Ann Oncol 26:iv1–iv100 (Supplement 4)
Lee M, Yee J, Kim JY et al (2019) Risk factors for neutropenia and febrile neutropenia following prophylactic pegfilgrastim. Asia Pac J Clin Oncol 15(4):231–237
Havrilesky LJ, Reiner M, Morrow PK et al (2015) A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol 93:203–210
Luciani A, Bertuzzi C, Ascione G et al (2009) Dose intensity correlate with survival in elderly patients treated with chemotherapy for advanced non-small cell lung cancer. Lung Cancer 66:94–96
Loibl S, Skacel T, Nekljudova V et al (2011) Evaluating the impact of relative total dose intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer- a pooled analysis. BMC Cancer 11:131
Hanna RK, Poniewierski MS, Laskey RA et al (2013) Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol 129:74–80
Lee JC, Kim JW, Ahn S et al (2017) Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: using cumulative relative dose intensity. Eur J Cancer 76:125–133
Kobayashi S, Ueno M, Omae K et al (2019) Influence of initial dose intensity on efficacy of FOLFIRINOX in patients with advanced pancreatic cancer. Oncotarget 10(19):1775–1784
Ozaka M, Ishii H, Sato T et al (2018) A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 81(6):1017–1023
Mahaseth H, Brutcher E, Kauh J et al (2013) Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 42(8):1311–1315
Stein SM, James ES, Deng Y et al (2016) Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 114(7):737–743
Kajiyama K, Ishiguro C, Ando T et al (2021) Nested case-control study utilizing MID-NET® on thrombocytopenia associated with pegfilgrastim in patients treated with antineoplastic agents. Clin Pharmacol Ther. https://doi.org/10.1002/cpt.2263 (Online ahead of print)
Masuda N, Tokuda Y, Nakamura S et al (2015) Dose response of pegfilgrastim in Japanese breast cancer patients receiving six cycles of docetaxel, doxorubicin, and cyclophosphamide therapy: a randomized controlled trial. Support Care Cancer 23(10):2891–2898
Faris JE, Blaszkowsky LS, McDermott S et al (2013) FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 18:543–548
Acknowledgements
We thank all the patients, their families, the investigators, co-medical staffs and all others who participated in the present study. This research was supported by JSPS KAKENHI Grant Number JP 26461039.
Funding
This research was supported by JSPS KAKENHI Grant Number JP 26461039.
Author information
Authors and Affiliations
Contributions
MS and HU contributed to the study conception and design. MS, HU, AO, HH, YS, SK, CM, TO, SK, MU, TT, MG, DI and SN were involved in data acquisition. MS and HU performed data analysis and interpretation. The first draft of the manuscript was written by MS, MI, SM, HU and TO. All the authors reviewed and commented on subsequent drafts of the manuscript. All the authors read and approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Conflict of interest
M. Ueno has received honoraria for speaking and research grants from Yakult Honsha and Taiho Pharmaceutical; C. Morizane has received research grants from Yakult Honsha and Taiho Pharmaceutical; M. Ikeda has received honoraria for speaking from Taiho Pharmaceutical and research grants from Yault Honsha; S. Mitsunaga has received honoraria for speaking from Pfizer Japan and research grants from Taiho Pharmaceutical; Y. Sakamoto has received research grants from Takeda Pharmaceutical; M. Sasaki, H. Ueno, A. Ohba, H. Hosoi, S. Kobayashi, T. Terazawa, M. Goto, D. Inoue, S. Namiki, S. Kondo and T. Okusaka have no conflict of interest to declare.
Ethical approval
This study was conducted in accordance with the principles laid down in the 1964 Declaration of Helsinki and its later amendments, and the protocol was approved by the Ethics Committee of National Cancer Center (Approval No. 2014-310) and all the institutions participating this study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sasaki, M., Ueno, H., Mitsunaga, S. et al. A phase II study of FOLFIRINOX with primary prophylactic pegfilgrastim for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Int J Clin Oncol 26, 2065–2072 (2021). https://doi.org/10.1007/s10147-021-02001-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02001-y