Abstract
Background
Colorectal cancer is common, and its incidence is increasing throughout the world. The liver is a major metastatic site, and colorectal liver metastasis (CRLM) has a poor prognosis. Although liver resection is the most effective therapy for CRLM, postoperative recurrence is common. Thus, prognostic markers for CRLM are greatly needed. D-dimer, a fibrin cleavage product, has been shown to be related to colorectal tumor progression, and is also associated with malignant progression and recurrence in various cancers. Therefore, we evaluated the value of D-dimer in predicting the prognosis in CRLM.
Methods
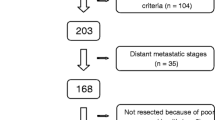
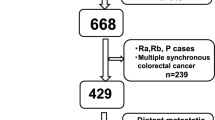
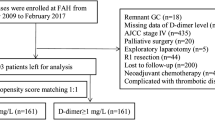
We retrospectively evaluated 90 cases of resected CRLM to determine the correlation between D-dimer and patient survival. The cut-off value for D-dimer levels was determined using receiver operating characteristic curve analysis.
Results
Significant differences occurred in the recurrence group with higher D-dimer levels (P = 0.00736*), while the optimal cut-off value was 0.6 µg/mL. High D-dimer levels (≥ 0.6 µg/mL) were associated with poor recurrence-free survival (RFS; P = 0.0000841*) and cancer-specific survival (CSS; P = 0.00615*). In the multivariate analysis, D-dimer correlated with CRLM prognosis and independently predicted RFS (P = 0.0179*).
Conclusion
High D-dimer levels were associated with poor RFS and CSS. D-dimer was an independent prognostic factor of RFS. Therefore, D-dimer may help predict recurrence and prognosis in patients with CRLM.




Similar content being viewed by others
Abbreviations
- CRLM:
-
Colorectal liver metastasis
- CEA:
-
Carcinoembryonic antigen
- ROC:
-
Receiver operating characteristic
- VTE:
-
Venous thrombosis
- NLR:
-
Neutrophil/lymphocyte ratio
- mGPS:
-
Modified Glasgow Prognostic Score
- RFS:
-
Recurrence-free survival
- CSS:
-
Cancer-specific survival
- CTCs:
-
Circulating tumor cells
References
Akgul O, Cetinkaya E, Ersoz S et al (2014) Role of surgery in colorectal cancer liver metastases. World J Gastroenterol 20(20):6113–6122
McNally SJ, Parks RW (2013) Surgery for colorectal liver metastases. Dig Surg 30(4–6):337–347
Simmonds PC, Primrose JN, Colquitt JL et al (2006) Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 94(7):982–999
Fong Y, Fortner J, Sun RL et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 230(3):309–318 (discussion 318–321)
Nagashima I, Takada T, Adachi M et al (2006) Proposal of criteria to select candidates with colorectal liver metastases for hepatic resection: comparison of our scoring system to the positive number of risk factors. World J Gastroenterol 12(39):6305–6309
Pulivarthi S, Gurram MK (2014) Effectiveness of d-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 6(10):491–499
Motavaf E, Sunesen KG, Stender MT et al (2014) Prognostic value of preoperative D-dimer and carcinoembryonic antigen levels in patients undergoing intended curative resection for colorectal cancer: a prospective cohort study. Int J Colorectal Dis 29(11):1427–1432
Yamamoto M, Yoshinaga K, Matsuyama A et al (2012) Plasma D-dimer level as a mortality predictor in patients with advanced or recurrent colorectal cancer. Oncology 83(1):10–15
Zhou YX, Yang ZM, Feng J et al (2013) High plasma D-dimer level is associated with decreased survival in patients with lung cancer: a meta-analysis. Tumour Biol 34(6):3701–3704
Ma X, Li Y, Zhang J et al (2014) Prognostic role of D-dimer in patients with lung cancer: a meta-analysis. Tumour Biol 35(3):2103–2109
Khoury JD, Adcock DM, Chan F et al (2010) Increases in quantitative D-dimer levels correlate with progressive disease better than circulating tumor cell counts in patients with refractory prostate cancer. Am J Clin Pathol 134(6):964–969
Go SI, Lee MJ, Lee WS et al (2015) D-dimer can serve as a prognostic and predictive biomarker for metastatic gastric cancer treated by chemotherapy. Med (Baltimore) 94(30):e951
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458
Zacharski LR, Wojtukiewicz MZ, Costantini V et al (1992) Pathways of coagulation/fibrinolysis activation in malignancy. Semin Thromb Hemost 18(1):104–116
Caine GJ, Lip GY, Stonelake PS et al (2004) Platelet activation, coagulation and angiogenesis in breast and prostate carcinoma. Thromb Haemost 92(1):185–190
Mytnik M, Stasko J (2011) D-dimer, plasminogen activator inhibitor-1, prothrombin fragments and protein C—role in prothrombotic state of colorectal cancer. Neoplasma 58(3):235–238
Edwards CM, Warren J, Armstrong L et al (1993) D-dimer: a useful marker of disease stage in surgery for colorectal cancer. Br J Surg 80(11):1404–1405
Kilic M, Yoldas O, Keskek M et al (2008) Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis 10(3):238–241
Xu G, Zhang YL, Huang W (2004) Relationship between plasma D-dimer levels and clinicopathologic parameters in resectable colorectal cancer patients. World J Gastroenterol 10(6):922–923
Blackwell K, Hurwitz H, Lieberman G et al (2004) Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer 101(1):77–82
Oya M, Akiyama Y, Yanagida T et al (1998) Plasma D-dimer level in patients with colorectal cancer: its role as a tumor marker. Surg Today 28(4):373–378
Mochizuki S, Yoshino T, Kojima T et al (2010) Therapeutic significance of a D-dimer cut-off level of > 3 microg/ml in colorectal cancer patients treated with standard chemotherapy plus bevacizumab. Jpn J Clin Oncol 40(10):933–937
Beppu T, Sakamoto Y, Hasegawa K et al (2012) A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 19(1):72–84
Inanc M, Er O, Karaca H et al (2013) D-dimer is a marker of response to chemotherapy in patients with metastatic colorectal cancer. J BUON 18(2):391–397
Zhu L, Liu B, Zhao Y et al (2014) High levels of D-dimer correlated with disease status and poor prognosis of inoperable metastatic colorectal cancer patients treated with bevacizumab. J Cancer Res Ther 10(Suppl):246–251
Tomimaru Y, Yano M, Takachi K et al (2008) Correlation between pretherapeutic d-dimer levels and response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. Dis Esophagus 21(4):281–287
Dirix LY, Vermeulen PB, Pawinski A et al (1997) Elevated levels of the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in sera of cancer patients. Br J Cancer 76(2):238–243
Sakamoto Y, Miyamoto Y, Beppu T et al (2015) Post-chemotherapeutic CEA and CA19-9 are prognostic factors in patients with colorectal liver metastases treated with hepatic resection after oxaliplatin-based chemotherapy. Anticancer Res 35(4):2359–2368
Zhang D, Yu M, Xu T et al (2013) Predictive value of serum CEA, CA19-9 and CA125 in diagnosis of colorectal liver metastasis in Chinese population. Hepatogastroenterology 60(126):1297–1301
Loupakis F, Yang D, Yau L et al (2015) Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 107(3):pii: dju427
Shen H, Yang J, Huang Q et al (2015) Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol 21(21):6470–6478
Kirwan CC, Clarke AC, Howell SJ et al (2016) PO-31—circulating tumour cells and hypercoagulability: a lethal relationship in metastatic breast cancer. Thromb Res 140(Suppl 1):S188
Seeberg LT, Brunborg C, Waage A et al (2017) Survival impact of primary tumor lymph node status and circulating tumor cells in patients with colorectal liver metastases. Ann Surg Oncol 24(8):2113–2121
Zhao R, Cai Z, Li S et al (2017) Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells from colorectal cancer. Oncotarget 8(6):9293–9302
Tieken C, Versteeg HH (2016) Anticoagulants versus cancer. Thromb Res 140(Suppl 1):S148–S153
Klerk CP, Smorenburg SM, Otten HM et al (2005) The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol 23(10):2130–2135
van Doormaal FF, Di Nisio M, Otten HM et al (2011) Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol 29(15):2071–2076
Rothwell PM, Wilson M, Elwin CE et al (2010) Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376(9754):1741–1750
Acknowledgements
We thank Ms. Yukie Saito, Ms. Tomoko Yano, Ms. Tomoko Ubukata, and Ms. Yuki Saka for their excellent assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Watanabe, A., Araki, K., Harimoto, N. et al. D-dimer predicts postoperative recurrence and prognosis in patients with liver metastasis of colorectal cancer. Int J Clin Oncol 23, 689–697 (2018). https://doi.org/10.1007/s10147-018-1271-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1271-x