Abstract
To assess whether monitoring brain tissue oxygen partial pressure (PbtO2) or employing intracranial pressure (ICP)/cerebral perfusion pressure (CCP)-guided management improves patient outcomes, including mortality, hospital length of stay (LOS), mean daily ICP and mean daily CCP during the intensive care unit(ICU)stay. We searched the Web of Science, EMBASE, PubMed, Cochrane Library, and MEDLINE databases until December 12, 2023. Prospective randomized controlled and cohort studies were included. A meta-analysis was performed for the primary outcome measure, mortality, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Eleven studies with a total of 37,492 patients were included. The mortality in the group with PbtO2 was 29.0% (odds ratio: 0.73;95% confidence interval [CI]:0.56–0.96; P = 0.03; I = 55%), demonstrating a significant benefit. The overall hospital LOS was longer in the PbtO2 group than that in the ICP/CPP group (mean difference:2.03; 95% CI:1.03–3.02; P<0.0001; I = 39%). The mean daily ICP in the PbtO2 monitoring group was lower than that in the ICP/CPP group (mean difference:-1.93; 95% CI: -3.61 to -0.24; P = 0.03; I = 41%). Moreover, PbtO2 monitoring did not improve the mean daily CPP (mean difference:2.43; 95%CI: -1.39 to 6.25;P = 0.21; I = 56%).Compared with ICP/CPP monitoring, PbtO2 monitoring reduced the mortality and the mean daily ICP in patients with severe traumatic brain injury; however, no significant effect was noted on the mean daily CPP. In contrast, ICP/CPP monitoring alone was associated with a short hospital stay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) refers to the destruction of the anatomy and physiology of the brain due to external forces. The estimated annual incidence of TBI is 27–69 million cases. Additionally, TBI is one of the leading causes of mortality and morbidity globally, contributing to a substantial economic burden on families and society [1, 2]. Secondary brain injury occurs after TBI due to high oxygen consumption and insufficient cerebral blood flow, which results in cerebral hypoxia. Currently, the treatment of TBI mainly focuses on the prevention of secondary brain injury with the routine monitoring of intracranial pressure (ICP) and maintaining adequate cerebral perfusion pressure (CPP) [3, 4] for the potential goal of maintaining a continuous supply of energy substrates and oxygen to improve neurological outcomes [5]. Currently, it is believed that ICP monitoring-guided treatment can improve the prognosis of patients with severe TBI. Moreover, the guidelines recommend monitoring ICP to calculate and maintain CPP to prevent cerebral ischemia and cerebral infarction [6,7,8]. However, studies have demonstrated that traditional ICP/ CPP monitoring cannot accurately detect most episodes of hypoxia [9]. Some patients with normal ICP and CPP may still develop cerebral ischemia and hypoxia [10].
Partial pressure of brain tissue oxygen (PbtO2) is a monitoring system for detecting the oxygen tension in the brain. Additionally, PbtO2 is an indicator that reflects the oxygenation status of the brain by directly measuring the partial pressure of oxygen in the brain using a probe placed within it. The normal range of PbtO2 is believed to be 16-40mmHg. Furthermore, PbtO2 of 10–15mmHg indicates mild cerebral hypoxia, and PbtO2 < 10mmHg indicates severe hypoxia. The purpose of PbtO2 is to identify episodes of decreased cerebral perfusion with or without associated raised ICP [11] because the partial pressure of oxygen in the brain can change in the early stages of injury [12]. Changes may also occur before or independently of increases in ICP [13]. PbtO2 monitoring has been used to assess, judge and regulate brain hypoxia to guide treatment and improve neurological outcomes [14]. PbtO2 can sensitively reflect the blood and oxygen supply to the brain, which is conducive to early detection of cerebral ischemia and hypoxia, and is an independent and sensitive predictor of cerebral ischemia and hypoxia [6]. Additionally, PbtO2 monitoring, ICP < 20mmHg, and CPP > 60mmHg have been reported to alleviate cerebral hypoxia through automatic regulation of the brain simultaneously [8].
However, the results of observational and cohort studies and randomized controlled trials remain controversial. Therefore, this meta-analysis was designed to summarize the effectiveness of this approach. The clinical efficacy and safety of PbtO2 in patients with TBI were evaluated by comparing the effects of PbtO2 (or combined) ICP/CPP-guided treatment and ICP/CPP-guided treatment alone on mortality, length of stay, ICP, and CPP during monitoring.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15] were followed in the review process and analyses. The study protocol was registered in PROSPERO (CRD42023494630).
Search strategy
The Web of Science, EMBASE, PubMed, Cochrane Library, MEDLINE databases were searched from inception to December 12, 2023. The search was performed using the following combination of keywords: brain injuries, traumatic or traumatic brain injury or TBI, and brain tissue oxygen or PbtO2 and monitoring. Search results were restricted to studies with adult participants published in English.
Inclusion and exclusion criteria
We used the following inclusion criteria: type of study (randomized or quasi-randomized controlled trials, cohort studies, or case-control studies); type of participants (studies with patients diagnosed with TBI); type of intervention (comparison of PbtO2 monitoring alone or combined PbtO2 and ICP/CPP monitoring with ICP/CPP monitoring alone); and type of outcome (primary outcome: mortality; secondary outcomes: length of stay in hospital, mean daily ICP, and mean daily CPP). Studies were excluded if they were published in languages other than English or as conference abstracts, case reports, or letters. Studies that included children; studies without sufficient data; and studies that reported data incorrectly (not appropriate for synthesis) were also excluded.
Study selection and data extraction
We removed duplicate articles from the search results using Endnote (version 9.3). The literature was then screened by two researchers (Yuqi Shen and Dan Wen) according to the inclusion and exclusion criteria outlined above. Disagreements were discussed with a third researcher (Zhenghua Liang) and a consensus was achieved. The extracted data included the first author, publication date, patient care settings, total sample size, intervention measures, and outcome indicators.
Quality evaluation
We assessed the quality of observational cohort studies using the Newcastle–Ottawa Scale (NOS). A score of 0–4 indicated low-quality literature (grade C), while 5–6 indicated medium-quality literature (grade B), and 7–9 indicated high-quality literature (grade A). The quality of randomized controlled trials was assessed using the Cochrane Risk of Bias Tool. A study was awarded grade A if the standards were completely met, grade B if the standards were partially satisfied, and grade C if none of the standards were met.
Statistical analysis
Statistical analysis was performed using Review Manager 5.4 (RevMan V5.4.1) provided by the Cochrane Collaboration (Oxford, UK). Odds ratios (ORs) with 95% confidence intervals (CIs) were determined to assess pooled effects. Heterogeneity in the included studies was assessed using Q and I2 tests. A random-effects model was adopted to analyze variations across the included studies. The stability of the results was assessed using sensitivity analysis by eliminating one study at a time.
Results
Study selection
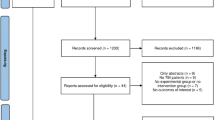
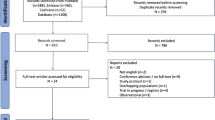
The PRISMA flowchart (Fig. 1) displays that 1862 articles were originally retrieved; 996 were retained after removing duplicates. During the first screening of titles and abstracts, 935 articles were excluded for the following reasons: irrelevant topic (538 articles), irrelevant population (145 articles), not reporting outcomes of interest (163 articles), case report (46 articles), or meta-analysis or systematic review (43 articles). Eleven articles that met all conditions were identified using full-text screening and included in the final analysis [13, 16,17,18,19,20,21,22,23,24,25].
Study characteristics and quality
The main features extracted from the studies that were included in our analysis are listed in Table 1. We included a total of 37,492 patients, with the sample size ranging from 50 to 35,501 participants per study. On quality assessment, six studies were awarded grade A [13, 18, 20,21,22,23], and four were awarded grade B [16, 17, 19, 24, 25]. Details of the assessment of each study are provided in Tables 2 and 3.
Primary outcome: mortality
The mortality rate was 29.0% in 2026 patients with TBI in the PbtO2 monitoring group. The OR for mortality in the PbtO2 monitoring group, compared to that of ICP/CPP, was 0.73 (95% CI: 0.56–0.96; P = 0.03; I2 = 55%), thus demonstrating a significant benefit (Fig. 2).
Secondary outcomes
The mean difference(MD)in the length of hospital stay in patients in the PbtO2 monitoring group (reported in six studies) was 2.03 (95% CI: 1.03–3.02; P<0.0001; I2 = 39%);therefore the overall length of stay in the hospital in the ICP/CPP monitoring group was shorter than that in the PbtO2 monitoring group (Fig. 3).
The MD in the mean daily ICP between PbtO2 monitoring group and ICP/CPP monitoring group was − 1.93 (95%CI: -3.61 to -0.24; P = 0.03; I2 = 41%)(based on five studies). The mean daily ICP in the PbtO2 monitoring group was lower than that in the ICP/CPP monitoring group (Fig. 4).
The MD in the mean daily CPP between the PbtO2 monitoring group and ICP/CPP monitoring group was 2.43 (95%CI: -1.39 to 6.25; P = 0.21; I2 = 56%), thus demonstrating a non-significant benefit(based on five studies). PbtO2 monitoring did not improve the mean daily CPP (Fig. 5).
Discussion
We pooled the results of nine retrospective cohort studies and two randomized controlled trials involving 37,492 patients with TBI, including 2,096 in the PbtO2 monitoring group and 35,396 in the ICP/CPP monitoring group. Our primary meta-analysis investigated the impact of brain tissue oxygen monitoring in patients with TBI compared to standard ICP/CPP monitoring in terms of mortality; hospital length of stay; and mean daily ICP and CPP.
Our results demonstrated that the role of PbtO2 monitoring in reducing mortality remains clear. Four studies included in this meta-analysis reported that PbtO2 monitoring does not decrease a significant mortality rate. In contrast, seven studies identified a reduction in the risk of mortality. The rate of mortality in the PbtO2 monitoring group was 29.0% across all studies included in our analysis, which is different from the previously reported rate based on a meta-analysis by Xie [4]. Studies have demonstrated that brain hypoxia is associated with poor prognosis after TBI and optimizing PbtO2 can improve recovery and survival rates [26]. Four observational studies [19, 20, 22, 23] and two randomized controlled trials [16, 25] included in this meta-analysis demonstrated that when PbtO2 was incorporated into clinical management decisions, neural function exhibits statistically significant benefits. Additionally, five studies demonstrated that PbtO2 monitoring increased mortality in patients with TBI. Although no single treatment has been identified to significantly decrease the mortality of these patients in a relatively short period, improving patient outcomes through corresponding interventions is possible.
PbtO2 monitoring group was associated with prolonged the hospital length of stay; however, it is worth noting that three reports included in this analysis revealed a significant difference in hospital length of stay, while three other studies indicated no such difference between patient groups. However, our meta-analysis results revealed that PbtO2 monitoring was associated with extended hospital length of stay. Although the severity of TBI at admission was comparable between patient groups, the difference may be attributed to patients in the PtbO2 monitoring group receiving more intensive life-support treatments, potentially leading to prolonged survival [18]. The relationship between PbtO2 monitoring and extended hospital length of stay in patients with TBI, therefore, needs further evaluation.
Only one study reported a significant relationship between the mean daily ICP and CPP with PbtO2 monitoring, while four studies reported no significant association. Our meta-analysis suggests that ICP can be reduced further with PbtO2 monitoring. However, mean daily CPP is demonstrated no statistical significance between the PbtO2 monitoring group and the ICP/CPP group. The discrepancy may stem from differences in the application and assessment methods of brain tissue oxygen monitoring and ICP/CPP between studies. We suggest that PbtO2 and ICP/CPP monitoring should be standardized, meanwhile, operators require better training to reduce the variations in results.
Limitations
This study has some limitations. First, this study included two randomized controlled trials, while the remaining studies were retrospective cohort studies. Cohort studies have a greater risk of bias than randomized controlled trials. Second, since these retrospective studies were not randomized, the characteristics of patients included in the two evaluated groups may have varied. Additionally, different operators on the application of tissue oxygen monitoring is also diverse. Third, some studies selected a historical cohort as a control, which may have affected the comparability between studies. However, high-quality randomized controlled trials can address these limitations.
Conclusions
In this systematic review and meta-analysis of 11 carefully selected studies, we compared PbtO2 monitoring with ICP/CPP monitoring in patients with TBI. Our analysis revealed that PbtO2 monitoring can decrease mortality and ICP but has no significant effect on and CPP. ICP/CPP monitoring appears to be associated with a short hospital length of stay, however, and we recommend this approach for patients with TBI as a potentially beneficial and effective intervention, as it allows for better monitoring and reduction of ICP contributing to a favorable neurological prognosis. The target population, intervention methods, implementation protocols, and high-quality randomized controlled trials are needed in large, well-designed studies and should be the focus of future research on the topic.
Data availability
No datasets were generated or analysed during the current study.
References
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV, Park KB (2018) Estimating the global incidence of traumatic brain injury. J Neurosurg 130(4):1080–1097. https://doi.org/10.3171/2017.10.JNS17352
GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators (2019) Lancet Neurol 18(1):56–87. https://doi.org/10.1016/S1474-4422(18)30415-0. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016
Eriksson EA, Barletta JF, Figueroa BE, Bonnell BW, Vanderkolk WE, McAllen KJ, Ott MM (2012) Cerebral perfusion pressure and intracranial pressure are not surrogates for brain tissue oxygenation in traumatic brain injury. Clin Neurophysiology: Official J Int Federation Clin Neurophysiol 123(6):1255–1260. https://doi.org/10.1016/j.clinph.2011.08.035
Xie Q, Wu HB, Yan YF, Liu M, Wang ES (2017) Mortality and outcome comparison between brain tissue oxygen combined with intracranial Pressure/Cerebral perfusion pressure-guided therapy and intracranial Pressure/Cerebral perfusion pressure-guided therapy in traumatic Brain Injury: a Meta-analysis. World Neurosurg 100:118–127. https://doi.org/10.1016/j.wneu.2016.12.097
Adamides AA, Cooper DJ, Rosenfeldt FL, Bailey MJ, Pratt N, Tippett N, Vallance S, Rosenfeld JV (2009) Focal cerebral oxygenation and neurological outcome with or without brain tissue oxygen-guided therapy in patients with traumatic brain injury. Acta Neurochir 151(11):1399–1409. https://doi.org/10.1007/s00701-009-0398-y
Narotam PK, Morrison JF, Nathoo N (2009) Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg 111(4):672–682. https://doi.org/10.3171/2009.4.JNS081150
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and, Care C, Bratton AANSCNS, Chestnut SL, Ghajar RM, McConnell J, Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW (2007) Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma 24(Suppl 1):S37–S44. https://doi.org/10.1089/neu.2007.9990
Figaji AA, Zwane E, Graham Fieggen A, Argent AC, Roux L, P. D., Peter JC (2010) The effect of increased inspired fraction of oxygen on brain tissue oxygen tension in children with severe traumatic brain injury. Neurocrit Care 12(3):430–437. https://doi.org/10.1007/s12028-010-9344-3
Gracias VH, Guillamondegui OD, Stiefel MF, Wilensky EM, Bloom S, Gupta R, Pryor JP, Reilly PM, Leroux PD, Schwab CW (2004) Cerebral cortical oxygenation: a pilot study. J Trauma 56(3):469–474. https://doi.org/10.1097/01.ta.0000114274.95423.c0
Chang JJ, Youn TS, Benson D, Mattick H, Andrade N, Harper CR, Moore CB, Madden CJ, Diaz-Arrastia RR (2009) Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med 37(1):283–290. https://doi.org/10.1097/CCM.0b013e318192fbd7
Shanahan R, Avsar P, Watson C, Moore Z, Patton D, McEvoy NL, Curley G, O’Connor T (2023) The impact of brain tissue oxygenation monitoring on the Glasgow Outcome Scale/Glasgow Outcome Scale Extended in patients with moderate to severe traumatic brain injury: a systematic review. Nurs Crit Care. https://doi.org/10.1111/nicc.12973Advance online publication
van den Brink WA, van Santbrink H, Steyerberg EW, Avezaat CJ, Suazo JA, Hogesteeger C, Jansen WJ, Kloos LM, Vermeulen J, Maas AI (2000) Brain oxygen tension in severe head injury. Neurosurgery 46(4):868–878. https://doi.org/10.1097/00006123-200004000-00018
Komisarow JM, Toro C, Curley J, Mills B, Cho C, Simo GM, Vavilala MS, Laskowitz DT, James ML, Mathew JP, Hernandez A, Sampson J, Ohnuma T, Krishnamoorthy V (2022) Utilization of Brain Tissue Oxygenation Monitoring and Association with Mortality following severe traumatic brain Injury. Neurocrit Care 36(2):350–356. https://doi.org/10.1007/s12028-021-01394-y
Hirschi R, Hawryluk GWJ, Nielson JL, Huie JR, Zimmermann LL, Saigal R, Ding Q, Ferguson AR, Manley G (2018) Analysis of high-frequency PbtO2 measures in traumatic brain injury: insights into the treatment threshold. J Neurosurg 1–11 Advance online publication. https://doi.org/10.3171/2018.4.JNS172604
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Lin, C. M., Lin, M. C., Huang, S. J., Chang, C. K., Chao, D. P., Lui, T. N., Ma, H. I., Liu, M. Y., Chung, W. Y., Shih, Y. H., Tsai, S. H., Chiou, H. Y., Lin, M. R., Jen, S. L., Wei, L., Wu, C. C., Lin, E. Y., Liao, K. H., Chiang, Y. H., Chiu, W. T., ? Lin, J. W. (2015). A Prospective Randomized Study of Brain Tissue Oxygen Pressure-Guided Management in Moderate and Severe Traumatic Brain Injury Patients. BioMed research international, 2015, 529580. https://doi.org/10.1155/2015/529580
Meixensberger J, Jaeger M, Väth A, Dings J, Kunze E, Roosen K (2003) Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry 74(6):760–764. https://doi.org/10.1136/jnnp.74.6.760
Barrit S, Al Barajraji M, Hadweh E, Dewitte S, Torcida O, Andre N, Taccone J, Schuind FS, S., Gouvêa Bogossian E (2022) Brain tissue oxygenation-guided therapy and outcome in traumatic Brain Injury: a single-Center Matched Cohort Study. Brain Sci 12(7):887. https://doi.org/10.3390/brainsci12070887
Spiotta AM, Stiefel MF, Gracias VH, Garuffe AM, Kofke WA, Maloney-Wilensky E, Troxel AB, Levine JM, Le Roux PD (2010) Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg 113(3):571–580. https://doi.org/10.3171/2010.1.JNS09506
Green JA, Pellegrini DC, Vanderkolk WE, Figueroa BE, Eriksson EA (2013) Goal directed brain tissue oxygen monitoring versus conventional management in traumatic brain injury: an analysis of in hospital recovery. Neurocrit Care 18(1):20–25. https://doi.org/10.1007/s12028-012-9797-7
Martini RP, Deem S, Yanez ND, Chesnut RM, Weiss NS, Daniel S, Souter M, Treggiari MM (2009) Management guided by brain tissue oxygen monitoring and outcome following severe traumatic brain injury. J Neurosurg 111(4):644–649. https://doi.org/10.3171/2009.2.JNS08998
McCarthy MC, Moncrief H, Sands JM, Markert RJ, Hall LC, Wenker IC, Anderson HL 3rd, Ekeh AP, Walusimbi MS, Woods RJ, Saxe JM, Tchorz KM (2009) Neurologic outcomes with cerebral oxygen monitoring in traumatic brain injury. Surgery 146(4):585–591. https://doi.org/10.1016/j.surg.2009.06.059
Hoffman H, Abi-Aad K, Bunch KM, Beutler T, Otite FO, Chin LS (2021) Outcomes associated with brain tissue oxygen monitoring in patients with severe traumatic brain injury undergoing intracranial pressure monitoring. J Neurosurg 135(6):1799–1806. https://doi.org/10.3171/2020.11.JNS203739
Stiefel MF, Spiotta A, Gracias VH, Garuffe AM, Guillamondegui O, Maloney-Wilensky E, Bloom S, Grady MS, LeRoux PD (2005) Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg 103(5):805–811. https://doi.org/10.3171/jns.2005.103.5.0805
Payen JF, Launey Y, Chabanne R, Gay S, Francony G, Gergele L, Vega E, Montcriol A, Couret D, Cottenceau V, Pili-Floury S, Gakuba C, Hammad E, Audibert G, Pottecher J, Dahyot-Fizelier C, Abdennour L, Gauss T, Richard M, Vilotitch A, Bosson JL, Bouzat P (2023) Intracranial pressure monitoring with and without brain tissue oxygen pressure monitoring for severe traumatic brain injury in France (OXY-TC): an open-label, randomised controlled superiority trial. Lancet Neurol 22(11):1005–1014. https://doi.org/10.1016/S1474-4422(23)00290-9. OXY-TC trial collaborators
Gouvêa Bogossian E, Diosdado A, Barrit S, Al Barajraji M, Annoni F, Schuind S, Taccone FS (2022) The impact of invasive brain oxygen pressure guided therapy on the outcome of patients with traumatic Brain Injury: a systematic review and Meta-analysis. Neurocrit Care 37(3):779–789. https://doi.org/10.1007/s12028-022-01613-0
Funding
This work was supported by Sichuan Hospital Management and Development Research Center [grant numbers SCYG2023-10] and Zigong City philosophy and social science key research base health humanities research center [grant numbers JKRWY23-21].
Author information
Authors and Affiliations
Contributions
Yuqi Shen, Dan Wen, Zhenhua Liang: Study design Li Wan, Qingli Jiang, Haiyan He: Data collection/analysis Yuqi Shen, Dan Wen, Mei He: Writing of first draft of the paper All authors: Revising of paper for important intellectual contentAll authors: Approval of final version.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, Y., Wen, D., Liang, Z. et al. Brain tissue oxygen partial pressure monitoring and prognosis of patients with traumatic brain injury: a meta-analysis. Neurosurg Rev 47, 222 (2024). https://doi.org/10.1007/s10143-024-02439-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02439-4