Abstract
Background
This study examines the effect of an increase in the inspired fraction of oxygen (FiO2) on brain tissue oxygen (PbO2) in children with severe traumatic brain injury (TBI).
Methods
A prospective observational study of patients who underwent PbO2 monitoring and an oxygen challenge test (temporary increase of FiO2 for 15 min) was undertaken. Pre- and post-test values for arterial partial pressure of oxygen (PaO2), PbO2, and arterial oxygen content (CaO2) were examined while controlling for any changes in arterial carbon dioxide tension and cerebral perfusion pressure during the test. Baseline transcranial Doppler studies were done. Outcome was assessed at 6 months.
Results
A total of 43 tests were performed in 28 patients. In 35 tests in 24 patients, the PbO2 monitor was in normal-appearing white matter and in eight tests in four patients, the monitor was in a pericontusional location. When catheters were pericontusional or in normal white matter the baseline PbO2/PaO2 ratio was similar. PaO2 (P < 0.0001) and PbO2 (P < 0.0001) significantly increased when FiO2 was increased. The magnitude of the PbO2 response (∆PbO2) was correlated with ∆PaO2 (P < 0.0001, R 2 = 0.37) and ∆CaO2 (P = 0.001, R 2 = 0.23). The ∆PbO2/∆PaO2 ratio (oxygen reactivity) varied between patients, was related to the baseline PbO2 (P = 0.001, r = 0.54) and was inversely related to outcome (P = 0.02, confidence interval 0.03–0.78).
Conclusion
Normobaric hyperoxia increases PbO2 in children with severe TBI, but the response is variable. The magnitude of this response is related to the change in PaO2 and the baseline PbO2. A greater response appears to be associated with worse outcome.
Similar content being viewed by others
Introduction
Brain tissue oxygen tension (PbO2) monitoring is used more frequently in adult traumatic brain injury (TBI) to supplement intracranial pressure (ICP) and cerebral perfusion (CPP) monitoring to guide treatment. Reduced PbO2 is associated with poor outcome and so management protocols to maintain PbO2 above a threshold of 15–20 mmHg are recommended [1–5]. The associations between PbO2 and outcome, and the determinants of PbO2, are less well studied in pediatric TBI [6, 7]. In particular, the relationship between arterial oxygen tension (PaO2) and PbO2 in children, and the implications of this relationship, have not been examined.
What determines PbO2 is only beginning to be elucidated [8]. In experimental and clinical studies, PbO2 is associated variably with systemic factors such as arterial saturation and blood pressure, and local factors such as ICP, CPP, cerebral blood flow (CBF), cerebral metabolic rate of oxygen, product of blood flow and oxygen content, mean transit time of blood through the brain, end-capillary venous oxygen tension, and arteriovenous difference of oxygen [8–16]. These studies suggest that PaO2 may strongly influence PbO2; however, the PbO2 response to increased PaO2 varies between patients. The determinants and significance of the magnitude of this PbO2 response are unclear and while normobaric hyperoxia may correct compromised PbO2 and possibly improve brain metabolism, its overall benefits are not known [17–19]. However, the benefits of hyperoxia for brain metabolism and PbO2 are not inevitable [20]. This is important since there is a well-described association between reduced PbO2 and poor outcome in adults and children [1–5] whereas hyperoxia is known to have potential deleterious side-effects to both the lungs and to the brain [21–24]. The effects of hyperoxia on PbO2 in pediatric TBI are unknown since studies that have examined this question to date are in adult patients. Therefore, in this study we examined children with severe TBI to determine (1) the relationship between increased PaO2 and PbO2, and (2) the association of this relationship with clinical outcome.
Methods and Materials
Patient Selection
Ethics approval for the study was obtained from the institutional review boards of the University of Cape Town and the Red Cross War Memorial Children’s Hospital. Consent was taken from the child’s closest relative for inclusion in the study.
Patient Selection
All patients considered for inclusion in the study received ICP and PbO2 monitors. This study was part of a larger prospective observational study of intracranial monitoring in children with TBI. In this study, we collected data from all consecutive children (age <15 years old) with severe TBI (Glasgow Coma Score ≤ 8) and who received a PbO2 monitor and an oxygen challenge test (OCT). OCTs were included in the study only if the patient (1) was hemodynamically stable, (2) had the PbO2 monitor in situ for more than 12 h, (3) had a stable PbO2 signal, (4) did not have vasospasm (diagnosed with Lindegaard’s ratio on transcranial Doppler, TCD), and (5) had a PbO2 catheter placed in normal-appearing white matter on the head CT scan.
ICP and PbO2 Monitors
ICP was measured with an intraparenchymal monitor [Codman ICP Express (Codman, Raynham, MA) or Camino (Integra Neurosciences, Plainsboro, NJ)]. When an ICP monitor was placed, we also monitored and treated PbO2. PbO2 monitors (Licox®, Integra Neurosciences, Plainsboro, NJ) were inserted into normal-appearing right frontal white matter if there were no localized lesions, or in the hemisphere with the greater swelling or containing focal lesions. The position of the monitor was confirmed on follow-up head computed tomography (CT). PbO2 catheters were allowed to stabilize and OCTs were included in the study only if performed >12 h after catheter insertion.
Patient Management
Patient care was based on a local protocol consistent with the current recommendations for the management of severe TBI in children. Briefly, all patients were resuscitated on admission, underwent immediate evacuation of space-occupying lesions when present, and were intubated and mechanically ventilated in the pediatric intensive care unit (ICU). Elevated ICP was treated using a stepwise approach when ICP was >20 mmHg according to the guidelines for ICP management in children [25, 26]. We aimed to keep CPP > 50 mmHg in children >2 years old, and >45 mmHg in children <2 years old. Compromised (low) PbO2 was defined as <20 mmHg and was treated using a hierarchical treatment algorithm based on the possible cause for low PbO2. Further details of our ICP and PbO2 management are described elsewhere [6].
Oxygen Challenge Tests
OCTs were part of regular clinical care to test the response of the PbO2 monitor and were performed by increasing the inspired fraction of oxygen (FiO2) setting on the ventilator for 15 min. Pre- and post-test data, including the PbO2 response, were collected. OCTs were included in this study if the following values were recorded: (1) pre- and post-test values for PbO2, ICP, CPP, PaO2, arterial saturation (SaO2), arterial partial pressure of carbon dioxide (PaCO2), and (2) baseline values for hemoglobin (Hb) and Transcranial Doppler (TCD) mean flow velocity of the middle cerebral artery (FVMCA) recorded from the hemisphere with the PbO2 monitor. TCD studies were performed using a standard TCD machine and 2 MHz probe (Smart-Lite™, Rimed, Raanana, Israel). The highest mean flow velocity obtained for the MCA was recorded. Principal analysis was performed only on OCT data obtained from PbO2 catheters placed in normal-appearing white matter on head CT scan because of different physiological changes in pericontusional locations. These results also were compared to data obtained from pericontusional PbO2 probes.
Physiological Data
An arterial sample was taken before the FiO2 increase (before test) and after 15 min at the higher FiO2 (after test). The oxygen content (CaO2) of arterial samples was calculated from the equation:
Differences between before and after OCT (after 15 min at the increased FiO2 level) values were recorded as ∆PbO2, ∆PaO2, ∆CaO2, ∆PaCO2, and ∆CPP. FiO2 was increased to 100% in most but not all patients. In the early part of this series, FiO2 was increased by 0.1 above the baseline for 15 min (n = 5 tests), while in the latter part of the series we adjusted our protocol to increase the FiO2 to 100% for 15 min (n = 38 tests). Because the baseline FiO2 also differed between patients, the ratio of the change in PbO2 for the change in PaO2 (∆PbO2/∆PaO2) was calculated to assess the response of PbO2 to the change in PaO2; therefore, data from all tests could be used. The ∆PbO2/∆PaO2 was termed the oxygen reactivity, and was used as the key variable against which other factors were tested for association.
Outcome
Clinical outcome was assessed at 6 months after injury using the Glasgow Outcome Score (GOS) which was dichotomized to unfavorable (GOS 1–3) and favorable (GOS 4–5) outcome. We chose the GOS since we have observed similar outcome results when using the dichotomized GOS or the dichotomized Pediatric Cerebral Performance Category Scale [4].
Statistical Analysis
All data were analysed with R statistical computing (http://wwww.r-project.org). Variables were tested for normality with the Shapiro–Wilk test. Differences between the before test and after test values for PaO2 and PbO2 were examined with the Wilcoxon’s rank sum test. The relationship between ∆PaO2 and ∆PbO2 was examined with Spearman’s correlation and Pearson’s product–moment correlation. Correlation coefficients are reported as r. Linear regression was used to examine the relationship between ∆PbO2 and ∆PaO2 and ∆CaO2 while controlling for ∆CO2 and ∆CPP. Since multiple studies in some patients were analysed, we used a general estimating equation (GEE) to account for inter-individual differences between tests with catheters placed in normal-appearing brain and those located near contusions for baseline values and ∆PbO2/∆PaO2 and for the analysis of data in Group A. Linear regression was used to examine the relationship between ∆PbO2/∆PaO2 and the following factors: baseline PbO2, baseline CPP, FVMCA, ∆CaO2, and day of testing (post-injury day; first 24 h = day 1). Coefficients for relationships between ∆PbO2/∆PaO2 and other variables were checked with nonparametric bootstrap methods to correct for repeat measures. ∆PbO2/∆PaO2 was further examined for relationships with outcome (using the GOS) and other parameters of PbO2 that were recorded for the whole duration of the monitoring period. This included the duration of time that PbO2 was <10 mmHg, the mean PbO2 in the first 24 h of monitoring (mPbO2 24) and the lowest PbO2 recorded for each patient. ∆PbO2/∆PaO2 also was examined with log transformation to ensure that the normality assumption was met. Results are expressed as mean ± SD or median and interquartile range (IQR) and range. Significance was set at P = 0.05.
Results
Patient Characteristics
A total of 43 tests in 28 patients were performed for which all required data were available. There were 24 patients (n = 35 tests) who had catheters placed in normal-appearing white matter (group A) and 4 patients (n = 8 tests) who had catheters placed close to contusions on (Group B). Head CT scans on these patients showed that the probe was placed close to the contusion but not obviously within it. Baseline variables for all patients are summarized in Table 1. For patients in Group A, baseline PbO2 was <15 mmHg at the time of testing in 3 of 35 tests (8.5%) and <10 mmHg in 1 (2.9%).
PbO2 Responses in Normal-Appearing Tissue (Group A) and Pericontusional Locations (Group B)
OCT results in Group A and B were tested for differences in PaO2, PbO2, PbO2/PaO2, ∆PbO2/∆PaO2, CPP, and FVMCA. The baseline FVMCA in Group B was slightly lower (P = 0.045) (Fig. 1). ∆PbO2/∆PaO2 was slightly higher in Group B (P = 0.049) but not when controlled for baseline PbO2/PaO2 (P = 0.055). There were no significant differences for PbO2 (P = 0.08), PbO2/PaO2 (P = 0.98), PaO2 (P = 0.07), or baseline CPP (P = 0.48) (Table 2). The remaining analysis below applies to Group A.
Effect of increased FiO2 on PaO2 and PbO2 (Group A)
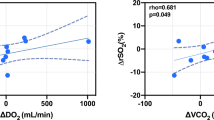
Baseline PaO2 and PbO2 were not significantly related (P = 0.3271). Induced hyperoxia significantly increased both PaO2 (P < 0.0001) and PbO2 (P < 0.0001). There was a significant relationship between ∆PbO2 and ∆PaO2 (P < 0.0001, R 2 = 0.371). This relationship was maintained when controlled for ∆CO2 and ∆CPP (P < 0.0001, R 2 = 0.412) during the test. ∆PbO2 and ∆CaO2 were also significantly related (P = 0.001, R 2 = 0.232).
Physiological Factors and ∆PbO2/∆PaO2 (Group A)
∆PbO2/∆PaO2 was significantly correlated with baseline PbO2 (Spearman’s r = 0.538, P = 0.001; linear regression P < 0.0001) (Fig. 2). Table 3 describes the relationships between ∆PbO2/∆PaO2 and other physiological factors. ∆PbO2/∆PaO2 was not correlated with baseline CPP (P = 0.27), baseline FVMCA (P = 0.44), or ∆CaO2 (P = 0.70). It was however, significantly correlated with the post-trauma day (r = 0.36, P = 0.03), i.e., greater ∆PbO2/∆PaO2 values were found with increasing day after injury, but not when adjusted for baseline PbO2 (P = 0.95). There were no significant relationships between ∆PbO2/∆PaO2 and other markers of low PbO2 for the duration of monitoring for each patient, including PbO2 < 10 mmHg, mean PbO2 for the first 24 h, and the lowest PbO2.
Outcome (Group A Patients; n = 24)
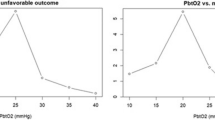
Outcome was as follows: GOS 1 [died] (n = 3, 12.5%); GOS 2 (n = 0); GOS 3 (n = 4, 16.7%); GOS 4 (n = 7, 29.2%); GOS 5 (n = 10, 41.7%). ∆PbO2/∆PaO2 was inversely related to dichotomized outcome, with and without log transformation of ∆PbO2/∆PaO2. This relationship remained significant when adjusted for baseline PbO2, day of testing and age (P = 0.02, 95% confidence interval 0.03–0.78) (Table 4). Therefore, a greater ∆PbO2/∆PaO2 was associated with a lower probability of favorable outcome.
Discussion
In this study, we examined the effect of a temporary increase of FiO2 on PbO2 in children with severe TBI. The main findings were: (1) induced normobaric hyperoxia significantly increased PbO2, (2) the magnitude of this increase was closely associated with the change in PaO2, (3) the response of PbO2 for the given change in PaO2 (∆PbO2/∆PaO2, or O2 reactivity) was increased when baseline PbO2 was higher, and (4) a greater PbO2 response to PaO2 change (higher ∆PbO2/∆PaO2) was associated with worse outcome.
Methodological Limitations
There are several potential limitations in this study. First, the sample size was small; therefore some significant relationships may not have been demonstrated; therefore these should be regarded as preliminary results. However, despite the limited sample size several significant results were found. The results of this study however, require replication in a larger study. Second, TCD-derived FVMCA was recorded but not local CBF. FVMCA is a good surrogate marker of changes of MCA CBF [27, 28] but does not necessarily reflect local CBF. Since PbO2 is a measure of local oxygen tension, local measures of CBF may have been better suited to this study. Third, the increase in FiO2 was not standardized. However, PaO2 varies in response even to a standard FiO2 increase and in this study PbO2 changes were interpreted relative to the magnitude of change in PaO2, not the FiO2 increase; therefore, the relative changes are valid for analysis. Fourth, we did not measure jugular venous saturation (SJVO2) and so we cannot comment on the relationship between PbO2 and the arteriovenous difference in oxygen content [8]. Fifth, we did not measure cerebral metabolism; therefore, we cannot comment on whether the observed increase in PbO2 is beneficial to the tissues. However, this has been addressed by others [17–20] and was not the aim of this study. Sixth, the duration of induced hyperoxia was 15 min; therefore, we cannot comment on the physiological effects of longer term changes in PbO2, potential adverse consequences of longer exposure to normobaric hyperoxia, or patient benefits. Seventh, there were relatively few tests performed when PbO2 was low (PbO2 was <10 mmHg in only one test). Since the PbO2 response to a FiO2 increase depends in part on the baseline PbO2 it is conceivable that whether PbO2 is compromised or not also may influence the results; this will require further study. Finally, the number of patients with poor outcome was relatively small; therefore, other factors related to outcome may not be apparent from these results. Despite these limitations, this is the first study to examine the effects of induced hyperoxia on PbO2 in children with severe TBI. The study demonstrates that hyperoxia consistently increases PbO2 but that this effect is variable, and in part is influenced by the baseline PbO2. The location of the catheter and length of time after injury may be contributing factors.
Determinants of the PbO2 Response
PbO2 was significantly increased by hyperoxia in this study; however, the PbO2 response relative to the change in PaO2 (∆PbO2/∆PaO2) challenge varied widely (2–61%). The magnitude of this response, however, was related to baseline PbO2. When baseline PbO2 was low, the corresponding response of PbO2 to hyperoxia was reduced. Rosenthal et al. [8, 29] demonstrated in adults an association between PbO2 and the achieved PaO2 with increased FiO2, which in turn was related to lung function. They observed a significant relationship between PbO2 and the product of CBF and cerebral arteriovenous oxygen tension difference. Even though they used a combination of global and local factors, their results suggested that PbO2 may be more indicative of oxygen diffusion rather than simple perfusion or oxygen delivery. This is consistent with the views of Menon et al. [30] and Bullock [31] that the increased oxygen tension in the tissue may overcome diffusion barriers by increasing the pressure gradient from the capillary to the cell. It is possible that the findings of our study that oxygen reactivity was less when PbO2 was low may be due mechanisms limiting tissue perfusion or oxygen diffusion that may be associated with low PbO2 and may also limit the response of the tissue to increased PaO2. This relationship between baseline PbO2 and oxygen reactivity is consistent with findings in adult studies [32, 33].
Outcome and the PbO2 Response to Increased FiO2
We observed an inverse relationship between a greater ∆PbO2/∆PaO2 and worse outcome. One possible explanation for this is that the regulation of local oxygen tension in the microcirculation may be altered in the more injured brain. The normal microvascular response to increased oxygen tension in the tissues is vasoconstriction [24, 34]; however, experimental stroke studies demonstrate that this phenomenon may be absent or even reversed in injured or penumbral tissue [13, 35]. A similar phenomenon also may explain different responses of PbO2 to changes in PaCO2 in injured versus noninjured tissue [36, 37]. Therefore, the increased response of PbO2 for a given change in PaO2 in this study may reflect an alteration of normal tissue regulatory mechanisms, which may in turn be associated with poor outcome. A similar finding was reported by Van Santbrink et al. [38] in adult TBI, in which patients with a lower O2 reactivity had better outcomes. We also observed a greater O2 reactivity in PbO2 catheters located in a pericontusional location, which may be consistent with the above theory. However, in this study a reduced response of PbO2 to an FiO2 increase was also associated with low baseline PbO2, and in pediatric and adult TBI low PbO2 has been reported to be associated with poor outcome [1, 4, 6, 39]. Hlatky et al. [32] found that patients with apparent ischemia had the lowest response to hyperoxia, suggesting that a poor response to hyperoxia would be associated with poor outcome. It is possible that both robust and poor PbO2 responses to hyperoxia may be associated with poor outcome, but are measures of different processes. Our data do not allow us to clarify this issue. Nevertheless our findings and those of others suggest that the PbO2 response to a “treatment” may be just as important to outcome as the threshold of PbO2 that indicates brain hypoxia.
Is There a Role for Normobaric Hyperoxia in TBI?
Our study shows that hyperoxia of short duration (15 min) can increase PbO2. Whether this improves brain metabolism or can benefit a child with severe TBI is not addressed by our study. The role of hyperoxia after TBI is unknown and subject to much debate. Furthermore hyperoxia, particularly when of long duration (>24 h), has several well-described potential deleterious effects on the lungs, brain, and cerebral blood vessels [40]. However, short periods of hyperoxia do not appear to increase oxidative stress [41, 42] and short-term exposure (<24 h) to normobaric hyperoxia is probably well tolerated with few adverse effects [35]. The side-effects, e.g., vasoconstriction, may not always mean “worse” since vasoconstriction in the brain may alleviate increased ICP or divert blood flow from “normal” to “impaired” tissue. Experimental studies have shown improved CBF and oxygenated Hb in ischemic core and penumbral tissue [35]. Different microvasculature responses to increased oxygen tension and a reduction in metabolic burden due to decreased peri-infarct depolarization may in part explain these findings.
Several clinical studies in adult patients with TBI have attempted to define the effect of normobaric hyperoxia on brain tissue metabolism, but the results have been conflicting [17–20, 43, 44]. It may be that the effect of hyperoxia depends in part on the nature of the ischemic insult. For example, in rats with ischemia induced by MCA occlusion, hyperoxia reduced infarct size and improved outcome [35, 45] while in dogs worse outcome was observed with hyperoxia after cardiac arrest [46]. Although hyperoxia increases tissue oxygen tension, it may not improve oxygen delivery to the brain, particularly under conditions of brain ischemia [47]. On the other hand, increased oxygen tension in the tissues may improve mitochondrial function in ways not yet fully understood [31, 48], such as an increased O2 pressure gradient which overcomes diffusion-limited tissue hypoxia [30] and the preferential use of dissolved oxygen for tissue oxygenation [49, 50]. It is our opinion that hyperoxia may have a role in TBI as a means to test PbO2 monitor function or as part of a cause-directed and stepwise approach to treatment for compromised PbO2 provided the response to the altered FiO2 can be measured. Whether this benefits the patient, and which methods of correcting PbO2 are best, will require further study.
Conclusion
Normobaric hyperoxia significantly increases PbO2 in children with severe TBI. There was a wide range in the PbO2 response to increased PaO2. The magnitude of the ∆PbO2/∆PaO2 response was significantly related only to baseline PbO2. Patients with a poor outcome had a greater PbO2 response to a PaO2 challenge, as did patients in whom the PbO2 catheter was in a pericontusional location, which may reflect disturbances of local circulatory responses to changes in PaO2.
References
Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103:805–11.
Meixensberger J, Jaeger M, Vath A, Dings J, Kunze E, Roosen K. Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2003;74:760–4.
Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26:1576–81.
Figaji AA, Zwane E, Thompson C, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury: Part 1: Relationship with outcome. Childs Nerv Syst. 2009;25(10):1325–33.
van den Brink WA, van Santbrink H, Steyerberg EW, et al. Brain oxygen tension in severe head injury. Neurosurgery 2000;46:868–76. discussion 876–878.
Figaji AA, Fieggen AG, Argent AC, Leroux PD, Peter JC. Does adherence to treatment targets in children with severe traumatic brain injury avoid brain hypoxia? A brain tissue oxygenation study. Neurosurgery. 2008;63:83–91, discussion 91–92.
Figaji AA, Zwane E, Thompson C, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury: Part 2: Relationship with clinical, physiological, and treatment factors. Childs Nerv Syst. 2009;25(10):1335–43.
Rosenthal G, Hemphill JC III, Sorani M, et al. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008;36:1917–24.
Doppenberg EM, Zauner A, Bullock R, Ward JD, Fatouros PP, Young HF. Correlations between brain tissue oxygen tension, carbon dioxide tension, pH, and cerebral blood flow—a better way of monitoring the severely injured brain? Surg Neurol. 1998;49:650–4.
Doppenberg EM, Zauner A, Watson JC, Bullock R. Determination of the ischemic threshold for brain oxygen tension. Acta Neurochir Suppl. 1998;71:166–9.
Jaeger M, Soehle M, Schuhmann MU, Winkler D, Meixensberger J. Correlation of continuously monitored regional cerebral blood flow and brain tissue oxygen. Acta Neurochir (Wien). 2005;147:51–6, discussion 56.
Valadka AB, Hlatky R, Furuya Y, Robertson CS. Brain tissue PO2: correlation with cerebral blood flow. Acta Neurochir Suppl. 2002;81:299–301.
Zauner A, Bullock R, Di X, Young HF. Brain oxygen, CO2, pH, and temperature monitoring: evaluation in the feline brain. Neurosurgery. 1995;37:1168–76, discussion 1176–1177.
Hemphill JC III, Smith WS, Sonne DC, Morabito D, Manley GT. Relationship between brain tissue oxygen tension and CT perfusion: feasibility and initial results. AJNR Am J Neuroradiol. 2005;26:1095–100.
Gupta AK, Hutchinson PJ, Fryer T, et al. Measurement of brain tissue oxygenation performed using positron emission tomography scanning to validate a novel monitoring method. J Neurosurg. 2002;96:263–8.
Scheufler KM, Lehnert A, Rohrborn HJ, Nadstawek J, Thees C. Individual value of brain tissue oxygen pressure, microvascular oxygen saturation, cytochrome redox level, and energy metabolites in detecting critically reduced cerebral energy state during acute changes in global cerebral perfusion. J Neurosurg Anesthesiol. 2004;16:210–9.
Magnoni S, Ghisoni L, Locatelli M, et al. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J Neurosurg. 2003;98:952–8.
Tolias CM, Reinert M, Seiler R, Gilman C, Scharf A, Bullock MR. Normobaric hyperoxia-induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J Neurosurg. 2004;101:435–44.
Nortje J, Coles JP, Timofeev I, et al. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: Preliminary findings. Crit Care Med. 2008;36:273–81.
Diringer MN, Aiyagari V, Zazulia AR, Videen TO, Powers WJ. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J Neurosurg. 2007;106:526–9.
Pagano A, Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci. 2003;1010:405–16.
Clark JM, Lambertsen CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971;23:37–133.
Nakajima S, Meyer JS, Amano T, Shaw T, Okabe T, Mortel KF. Cerebral vasomotor responsiveness during 100% oxygen inhalation in cerebral ischemia. Arch Neurol. 1983;40:271–6.
Floyd TF, Clark JM, Gelfand R, et al. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol. 2003;95:2453–61.
Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S65–7.
Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 6. Threshold for treatment of intracranial hypertension. Pediatr Crit Care Med. 2003;4:S25–7.
Steiner LA, Coles JP, Johnston AJ, et al. Assessment of cerebrovascular autoregulation in head-injured patients: a validation study. Stroke. 2003;34:2404–9.
Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17:913–5.
Rosenthal G, Hemphill JC, Sorani M, et al. The role of lung function in brain tissue oxygenation following traumatic brain injury. J Neurosurg. 2008;108:59–65.
Menon DK, Coles JP, Gupta AK, et al. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32:1384–90.
Bullock MR. Hyperoxia: good or bad? J Neurosurg. 2003;98:943–4, discussion 944.
Hlatky R, Valadka AB, Gopinath SP, Robertson CS. Brain tissue oxygen tension response to induced hyperoxia reduced in hypoperfused brain. J Neurosurg. 2008;108:53–8.
Longhi L, Valeriani V, Rossi S, De Marchi M, Egidi M, Stocchetti N. Effects of hyperoxia on brain tissue oxygen tension in cerebral focal lesions. Acta Neurochir Suppl. 2002;81:315–7.
Nishimura N, Iwasaki K, Ogawa Y, Shibata S. Oxygen administration, cerebral blood flow velocity, and dynamic cerebral autoregulation. Aviat Space Environ Med. 2007;78:1121–7.
Shin HK, Dunn AK, Jones PB, et al. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–42.
Dings J, Meixensberger J, Amschler J, Hamelbeck B, Roosen K. Brain tissue pO2 in relation to cerebral perfusion pressure, TCD findings and TCD-CO2-reactivity after severe head injury. Acta Neurochir (Wien). 1996;138:425–34.
Gupta AK, Hutchinson PJ, Al-Rawi P, et al. Measuring brain tissue oxygenation compared with jugular venous oxygen saturation for monitoring cerebral oxygenation after traumatic brain injury. Anesth Analg. 1999;88:549–53.
van Santbrink H, vd Brink WA, Steyerberg EW, Carmona Suazo JA, Avezaat CJ, Maas AI. Brain tissue oxygen response in severe traumatic brain injury. Acta Neurochir (Wien). 2003;145:429–38. discussion 438.
Maloney-Wilensky E, Gracias V, Itkin A, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med. 2009;37:2057–63.
Shah CV, Localio AR, Lanken PN, et al. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med. 2008;36:2309–15.
Doppenberg EM, Rice MR, Di X, Young HF, Woodward JJ, Bullock R. Increased free radical production due to subdural hematoma in the rat: effect of increased inspired oxygen fraction. J Neurotrauma. 1998;15:337–47.
Puccio AM, Hoffman LA, Bayir H, et al. Effect of short periods of normobaric hyperoxia on local brain tissue oxygenation and cerebrospinal fluid oxidative stress markers in severe traumatic brain injury. J Neurotrauma. 2009. doi:10.1089/neu.2008-0624.
Reinert M, Barth A, Rothen HU, Schaller B, Takala J, Seiler RW. Effects of cerebral perfusion pressure and increased fraction of inspired oxygen on brain tissue oxygen, lactate and glucose in patients with severe head injury. Acta Neurochir (Wien). 2003;145:341–9. discussion 349–350.
Reinert M, Schaller B, Widmer HR, Seiler R, Bullock R. Influence of oxygen therapy on glucose-lactate metabolism after diffuse brain injury. J Neurosurg. 2004;101:323–9.
Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–84.
Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006;26:821–35.
Rossi S, Stocchetti N, Longhi L, et al. Brain oxygen tension, oxygen supply, and oxygen consumption during arterial hyperoxia in a model of progressive cerebral ischemia. J Neurotrauma. 2001;18:163–74.
Scheufler KM, Rohrborn HJ, Zentner J. Does tissue oxygen-tension reliably reflect cerebral oxygen delivery and consumption? Anesth Analg. 2002;95:1042–8.
Suttner S, Piper SN, Kumle B, et al. The influence of allogeneic red blood cell transfusion compared with 100% oxygen ventilation on systemic oxygen transport and skeletal muscle oxygen tension after cardiac surgery. Anesth Analg. 2004;99:2–11.
Habler OP, Kleen MS, Hutter JW, et al. Effects of hyperoxic ventilation on hemodilution-induced changes in anesthetized dogs. Transfusion. 1998;38:135–44.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Figaji, A.A., Zwane, E., Graham Fieggen, A. et al. The Effect of Increased Inspired Fraction of Oxygen on Brain Tissue Oxygen Tension in Children with Severe Traumatic Brain Injury. Neurocrit Care 12, 430–437 (2010). https://doi.org/10.1007/s12028-010-9344-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-010-9344-3