Abstract
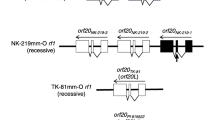
In soybean, the W4 gene encoding dihydroflavonol-4-reductase controls anthocyanin pigment biosynthesis in flowers. The mutant allele, w4-m, is characterized by variegated flowers and was evolved from the insertion of an endogenous transposable element, Tgm9, in intron II of the W4 gene. In the w4-m mutant line, reversion of the unstable allele from variegated to normal purple flower in revertants would indicate Tgm9’s excision accompanied by its insertion into a second locus. We identified a male-sterile, female-sterile mutant from such germinal revertant bearing purple flowers. The objectives of our investigation were to map the sterility locus, identify candidate genes for the male-fertile, female-fertile phenotype, and then determine if sterility is associated with the insertion of Tgm9 in the sterility locus. We used bulked segregant analysis to map the locus to molecular linkage group J (chromosome 16). Fine mapping enabled us to flank the locus to a 62-kb region that contains only five predicted genes. One of the genes in that region, Glyma16g07850.1, codes for a helicase. A rice homolog of this gene has been shown to control crossing over and fertility phenotype. Thus, Glyma16g07850.1 is most likely the gene regulating the male and female fertility phenotype in soybean. DNA blot analysis of the segregating individuals for Tgm9 showed perfect association between sterility and the presence of the transposon. Most likely, the sterility mutation was caused by the insertion of Tgm9. The transposable element should facilitate identification of the male- and female-fertility gene. Characterization of the fertility gene will provide vital molecular insight on the reproductive biology of soybean and other plants.


Similar content being viewed by others
References
Borner GV, Kleckner N, Hunter N (2004) Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117:29–45
Chen X-F, Palmer RG (1998) Instability at the k2 Mdh1-n y20 chromosomal region in soybean. Mol Gen Genet 260:309–318
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Groose RW, Schulte SM, Palmer RG (1990) Germinal reversion of an unstable mutation for anthocyanin pigmentation in soybean. Theor Appl Genet 79:161–167
Groose RW, Weigelt HD, Palmer RG (1988) Somatic analysis of an unstable mutation for anthocyanin pigmentation in soybean. J Hered 79:263–267
Guard AT (1931) Development of floral organs of the soy bean. Bot Gaz 91:97–102
He MY, Zhou YY, Xu ZR, Zhang JS (1979) The microsporogenesis and megasporogenesis of soybean. Acta Botanica Sinica 21:157–163
Johns CW, Delannay X, Palmer RG (1981) Structural sterility controlled by nuclear mutations in angiosperms. Nucleus 24:97–105
Joshi T, Patil K, Fitzpatrick MR, Franklin LD, Yao Q, Cook JR, Wang Z, Libault M, Brechenmacher L, Valliyodan B, Wu X, Cheng J, Stacey G, Nguyen HT, Xu D (2012) Soybean Knowledge Base (SoyKB): a web resource for soybean translational genomics. BMC genomics 13 1(Supp l):S15
Kato I, Sakaguchi S, Naito Y (1954) Development of flower parts and seed in the soybean plant, Glycine max M. Tokai Kinki Natn Agric Exptl Stn (Japan) Bull 1:96–114
Kato KK, Palmer RG (2003) Molecular mapping of the male-sterile, female-sterile mutant gene (st8) in soybean. J Hered 94:425–428
Kato KK, Palmer RG (2004) Molecular mapping of four ovule lethal mutants in soybean. Theor Appl Genet 108:577–585
Kaul MLH (1988) Male sterility in higher plants. Springer, Berlin
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Marmagne A, Ferro M, Meinnel T, Bruley C, Kuhn L, Garin J, Barbier-Brygoo H, Ephritikhine G (2007) A high content in lipid-modified peripheral proteins and integral receptor kinases features in the Arabidopsis plasma membrane proteome. Mol Cell Proteomics 6:1980–1996
Matsumoto T, Tanaka T, Sakai H, Amano N, Kanamori H, Kurita K, Kikuta A, Kamiya K, Yamamoto M, Ikawa H, Fujii N, Hori K, Itoh T, Sato K (2011) Comprehensive sequence analysis of 24,783 barley full-length cDNAs derived from 12 clone libraries. Plant Physiol 156:20–28
Mercier R, Jolivet S, Vezon D, Huppe E, Chelysheva L, Giovanni M, Nogue F, Doutriaux MP, Horlow C, Grelon M, Mezard C (2005) Two meiotic crossover classes cohabit in Arabidopsis: one is dependent on MER3, whereas the other one is not. Curr Biol 15:692–701
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Miksche JP (1961) Developmental vegetative morphology of Glycine max. Agron J 53:121–128
Palmer RG, Groose RW, Weigelt HD, Miller JE (1990) Registration of a genetic stock (w4-m w4-m) for unstable anthocyamin pigmentation in soybean. Crop Sci 30:1376–1379
Palmer RG, Hedges BR, Benavente RS, Grosse RW (1989) The w4-mutable line in soybean. Dev Genet 10:542–551
Palmer RG, Pfeiffer TW, Buss GR, Kilen TC (2004) Qualitative genetics. In: Boerma HR, Specht J (eds) Soybeans: improvement, production, and uses, 3rd edn. Agronomy Monograph no 16. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America Madison, WI, pp 137–233
Palmer RG, Sandhu D, Curran K, Bhattacharyya MK (2008a) Molecular mapping of 36 soybean male-sterile, female-sterile mutants. Theor Appl Genet 117:711–719
Palmer RG, Zhang L, Huang Z, Xu M (2008b) Allelism and molecular mapping of soybean necrotic root mutants. Genome 51:243–250
Rabinowicz T, Petetot JM, Khoury JC, de Courten-Myers GM (2009) Neocortical maturation during adolescence: change in neuronal soma dimension. Brain Cogn 69:328–336
Sandhu D, Gao H, Cianzio S, Bhattacharyya MK (2004) Deletion of a disease resistance nucleotide-binding-site leucine-rich-repeat-like sequence is associated with the loss of the Phytophthora resistance gene Rps4 in soybean. Genetics 168:2157–2167
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du J, Tian Z, Zhu L, Gill N, Joshi T, Libault M, Sethuraman A, Zhang XC, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Slattery RA, Pritzl S, Reinwand K, Trautschold B, Palmer RG, Sandhu D (2011) Mapping eight male-sterile, female-sterile soybean mutants. Crop Sci 51:231–236
Song QJ, Jia GF, Zhu YL, Grant D, Nelson RT, Hwang EY, Hyten DL, Cregan PB (2010) Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1.0) in Soybean. Crop Sci 50:1950–1960
Song QJ, Marek LF, Shoemaker RC, Lark KG, Concibido VC, Delannay X, Specht JE, Cregan PB (2004) A new integrated genetic linkage map of the soybean. Theor Appl Genet 109:122–128
Town SC, Putman CT, Turchinsky NJ, Dixon WT, Foxcroft GR (2004) Number of conceptuses in utero affects porcine fetal muscle development. Reproduction 128:443–454
Wang K, Tang D, Wang M, Lu J, Yu H, Liu J, Qian B, Gong Z, Wang X, Chen J, Gu M, Cheng Z (2009) MER3 is required for normal meiotic crossover formation, but not for presynaptic alignment in rice. J Cell Sci 122:2063–2088
Xu M, Brar HK, Grosic S, Palmer RG, Bhattacharyya MK (2010) Excision of an active CACTA-like transposable element from DFR2 causes variegated flowers in soybean [Glycine max (L.) Merr.]. Genetics 184:53–63
Xu M, Palmer RG (2005a) Genetic analysis and molecular mapping of a pale flower allele at the W4 locus in soybean. Genome 48:334–340
Xu M, Palmer RG (2005b) Molecular mapping of k2 Mdh1-n y20, an unstable chromosomal region in soybean [Glycine max (L.) Merr.]. Theor Appl Genet 111:1457–1465
Acknowledgments
We express our sincerest thanks to Dr. Karin Bodensteiner for critically reading the manuscript, her helpful comments, and encouraging remarks. Funding has been received from the UWSP Undergraduate Education Initiative fund, UWSP Student Research Fund, and USDA, Agricultural Research Service, and USDA-NRI.
Author information
Authors and Affiliations
Corresponding author
Additional information
This is a joint contribution from the Department of Biology, University of Wisconsin-Stevens Point, and the Department of Agronomy, Iowa State University. The mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the Department of Biology, UWSP, or Iowa State University, and the use of the name by the Department of Biology, UWSP, and Iowa State University implies no approval of the product to the exclusion of others that may also be suitable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1
Heatmap of RNA-seq analysis of the five candidate genes at different developmental stages. RFKM reads per kilobase of transcript per million mapped reads (PPTX 75 kb)
Supplemental Fig. S2
DNA gel blot analysis of plants segregating for fertility and sterility phenotypes. The 5′ end of the Tgm9 transposon was used as a probe. Plants were scored for fertility and sterility and fertile plants were progeny tested to score homozygous fertile and heterozygous plants. Fertility/sterility phenotype is shown on the top of the gel. A homozygous fertile, B homozygous sterile, H heterozygous. The arrow represents a band that is missing in all the homozygous fertile plants and is always present in homozygous sterile plants. Scoring for that band is written at the bottom of the gel. B/H band present, A band missing (PPTX 62 kb)
Rights and permissions
About this article
Cite this article
Raval, J., Baumbach, J., Ollhoff, A.R. et al. A candidate male-fertility female-fertility gene tagged by the soybean endogenous transposon, Tgm9. Funct Integr Genomics 13, 67–73 (2013). https://doi.org/10.1007/s10142-012-0304-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-012-0304-1