Abstract
Diet is one of the most important external factor shaping the composition and metabolic activities of the gut microbiome. The gut microbiome plays a crucial role in host health, including immune system development, nutrients metabolism, and the synthesis of bioactive molecules. In addition, the gut microbiome has been described as critical for the development of several mental disorders. Nutritional psychiatry is an emerging field of research that may provide a link between diet, microbial function, and brain health. In this study, we have reviewed the influence of different diet types, such as Western, Mediterranean, vegetarian, and ketogenic, on the gut microbiota composition and function, and their implication in various neuropsychiatric and psychological disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For several decades, many dietary patterns have been developed to improve the quality of life and health of people in our society. Dietary patterns constitute the quantity and the variety of foods in a diet, as well as the frequency with which the edibles are ingested. Some of the main dietary variants are (i) high-calorie diets (e.g., Western diet); (ii) mixed-balanced diets (e.g., Mediterranean diet); (iii) plant-based diets (e.g., vegetarian diet); and (iv) low-carbohydrate diets (e.g., ketogenic diet). These diets have different effects on physiological wellness, and some of them share common elements (Clemente-Suárez et al. 2023; Guasch-Ferré and Willett 2021). In spite of that, adherence to a particular dietary pattern depends, ultimately, on geographical, cultural, ethical and environmental awareness, self-image and physical fitness, health maintenance, and psychological well-being (Hargreaves et al. 2023; Tosti et al. 2018; Westman et al. 2003).
The variation of a healthy gut microbiome is considered as a physiological phenomenon that depends on age, ethnicity, lifestyle, and dietary habits (Valdes et al. 2018). According to Gilbert and Lynch (2019), the human-microbial co-dependence is subject to a co-evolution between the human immune system and the nutritional adaptation of the gut microbiome to the available nutrient substrates. The gut constitutes the most important human microbiome, which is not a stable and static system, but a dynamic and functional community that can change in space and time depending on the host physiological factors (Gerber 2014). Although “microbiota” and “microbiome” are often used interchangeably and synonymously, there are some differences between the two terms. The term microbiome encompasses a whole range of microorganisms, including bacteria, archaea, viruses, and fungi, the collection of genomes from all the microorganisms in that environment, as well as microbial structural elements, metabolites, and environmental conditions. The term microbiota is more restricted and describes the group of commensalist, symbiotic, and pathogenic microorganisms found in a given environment (Berg et al. 2020). Numerous studies have shown that the healthy human gut bacteriome in adults consists of 12 bacterial phyla (of which more than 93% belong to Actinomycetota, Bacillota, Bacteroidota, and Pseudomonadota); 18 bacterial families, the most prevalent being Bacteroidaceae (65.6%), Lachnospiraceae (11.5%), and Ruminococcaceae (8.4%); and 59 bacterial genera, with Bacteroides as the most abundant (more than 65%) (Almonacid et al. 2019; Costea et al. 2018; Nishijima et al. 2016). In addition, more than 3.8 million of microbial genes have been characterized, a proportion 150 times greater than the entire human genome, of which 99% are of bacterial origin (Li et al. 2014).
The gut-brain axis refers to a two ways communication network that connects the nervous system (central [CNS]; autonomic [ANS]; enteric [ENS]), or the hypothalamic–pituitary–adrenal (HPA) axis, with the intestine via vagal, neuroimmune, and neuroendocrine pathways (Cryan et al. 2019). Intermediate metabolites of the gut microbiome (e.g., short-chain fatty acids [SCFAs] and tryptophan catabolites), cytokines, and neurotransmitters, may be involved in various brain functions (Cryan et al. 2019; O’Mahony et al. 2015; Sandhu et al. 2017). Thus, the gut microbiome also plays a key role in the development of several neuropsychiatric disorders (Borrego-Ruiz and Borrego 2024; Cryan and Dinan 2015; Cryan et al. 2020; Lach et al. 2018).
Dietary patterns are one of the most important determinants of health, exerting their effects through mechanisms such as inflammation, oxidative stress, tryptophan metabolism, epigenetics, CNS and HPA functions, mitochondria, and gut microbiota (Marx et al. 2021; Rinninella et al. 2019). Furthermore, epidemiological studies have shown the involvement of dietary factors in the development of some mental disorders (Jacka et al. 2017; Marx et al. 2021; Ota et al. 2019; Parletta et al. 2019; Sánchez-Villegas et al. 2013), as well as their use as potential therapeutic tools (Adams et al. 2018; Estruch et al. 2018; Jacka et al. 2017). However, only a few studies have established a causal effect of the gut microbiome through dietary factors in mental disorders (Horn et al. 2022).
Based on the influence that diet exerts on gut microbiome composition and function, and also considering the existence of a network of interacting gut-brain communication, it has become clear that the interactions between the three components play a pivotal role in human health. Therefore, in this study, we have reviewed the influence of different diet types, such as traditional dietary habits (Western and Mediterranean diets) and emergent dietary habits (vegetarian and ketogenic diets), on the composition and function of the gut microbiome, and its implication in several mental disorders.
Method
The present work consists of a narrative review, aimed at collecting and analyzing the existing literature in order to provide a complete and exhaustive overview of the central topic of study. Both authors independently performed a conscientious literature search within the field that matched the topic under investigation. For this purpose, PubMed, Scopus, and Web of Science were examined, between August and September 2023, using different combinations of keywords related to the research object, such as “human gut microbiome”, “diets”, or “mental disorders”. The search strategy also included examining reference list from previous reviews and research papers. Both authors separately evaluated all suitable records, considering studies mostly focused on humans that explored the effect of diet on the gut microbiome and its implication in various neuropsychiatric and psychological disorders. Concerning that, each article found was individually assessed for its pertinence through the screening of title and abstract at first. Duplicate entries were removed, and as well those studies that were not likely to be included in the review due to their subject matters. The full texts of the remaining articles were meticulously inquired, and relevant data from them was extracted for further analysis. In this respect, studies that lacked significant information regarding the relationship between human gut microbiome and diet were excluded from the review, and also those ones that were meta-analyses, based on endocrine disturbances, and related to physiological, self-immune, or viral diseases.
Dietary types and their influence on the gut microbiome
Diets provide certain nutrients involved in the growth kinetics of gut bacteria, like glycans (inulin, lignin, pectin, cellulose, and fructooligosaccharides), which are indigestible carbohydrates for animals and humans (Cantarel et al. 2012). However, certain gut bacteria, known as primary degraders, which include members of the genera Bacteroides, Bifidobacterium and Ruminococcus, can break down glycans (Eilam et al. 2014). Moreover, diet can influence the metabolism and immune system of the host, and thus, modulate the shape of the microbiome through various substances, such as indole-derived compounds, vitamins A and D, and polyunsaturated fatty acids (Hibberd et al. 2017; Luthold et al. 2017).
Traditional dietary habits
Western diet (WD) is characterized by a high content of refined sugars, salt, saturated fats (especially omega-6 fatty acids), and proteins of animal origin (Varlamov 2017). This diet has been related to the occurrence of metabolic and pathological disorders, like type 2 diabetes, obesity, some cancers, and cardiovascular diseases (Clemente-Suárez et al. 2023; Mehta et al. 2017; Piernas et al. 2022).
The gut microbiota profile of individuals fed with a WD is similar to that found in obese individuals (David et al. 2014). A high-fat diet, which contains derived saturated fats, induces a decrease in Bacteroidota levels and an increase in members of both Pseudomonadota and Bacillota phyla in the gut microbiota (Bisanz et al. 2019; Malinowska et al. 2022). Furthermore, WD is rich in animal protein and provokes an increase of Bacteroides spp., Alistipes spp., and Bilophila spp., and a decrease in the levels of Lactobacillus spp., Enterococcus spp., Roseburia spp., and Eubacterium spp. (Beam et al. 2021; Muscogiuri et al. 2019; Singh et al. 2017).
The typical WD is associated with chronic inflammation, metabolic syndrome (diabetes, hypertension, and cardiovascular disease), and obesity (Cani 2013; Zinöcker and Lindseth 2018), due to the production of high levels of lipopolysaccharide (LPS) and trimethylamine N-oxide (TMAO) by the altered gut microbiota, and also to the decrease in SCFAs (Singh et al. 2017).
On the other hand, the Mediterranean diet (MD) consists mainly of important sources of fiber (cereals, nuts, legumes, vegetables, and fruits), unsaturated fatty acids, and antioxidant compounds (vitamins, flavonoids, phytosterols, minerals, terpenes, and polyphenols), with moderate consumption of eggs, white meat and fish or seafood, and low consumption of red meat and sweets (Estruch et al. 2018; Nagpal et al. 2019a). Compared to WD, MD has beneficial effects on human health and longevity, reducing the risk of cardiovascular disease, cancer, obesity, and other related metabolic disorders (Herpich et al. 2022; Lopez-Legarrea et al. 2014; Marlow et al. 2013; Muscogiuri et al. 2022; Richardson et al. 2022).
Such dietary adherence leads to a reshaped gut microbiota composition and provokes a higher microbial diversity (De Filippis et al. 2016; Garcia-Mantrana et al. 2018; Mitsou et al. 2017), with an increased population of the bacterial families Clostridiaceae and Lactobacillaceae, and of the genera Bacteroides, Bifidobacterium, Clostridium, Faecalibacterium, Lactobacillus, Oscillospira, Prevotella, and Roseburia, and also with a decrease in members of the phyla Pseudomonadota and Bacillota, and the genera Coprococcus and Ruminococcus (De Filippis et al. 2016; Garcia-Mantrana et al. 2018; Ghosh et al. 2020; Haro et al. 2017; Nagpal et al. 2019a; Pagliai et al. 2020).
Emergent dietary habits
Vegetarianism consists of a diet free of animal meat, characterized by low-fat and high-fiber components. Although diets based on vegetarianism are considered as emerging diets specially in Western countries, they have been a dietary pattern that dates back to ancient times in diverse cultures, mostly in Eastern geographic environments. There are different variants of vegetarian diets (VD), such as those that include dairy products and eggs (lacto-ovo-vegetarianism); those that include dairy products, but not eggs (lacto-vegetarianism); those that include eggs, but not dairy products (ovo-vegetarianism); and those that are strictly vegan, which do not include any derivative components of animal origin (Parker and Vadiveloo 2019; Xiao et al. 2022).
Several studies have reported a strong association between VD and certain health indicators, such as lower body fat index, lower incidence of type 2 diabetes, lower risk of suffering from cardiovascular disease, lower likelihood of developing various types of cancer, and increased longevity, compared to an omnivorous diet (Craig et al. 2021; Lassale et al. 2015; McMacken and Shah 2017; Olfert et al. 2022; Orlich et al. 2013; 2015; Pawlak 2017).
Adopting a VD reduces β-diversity (the amount of differentiation between species communities) of the gut microbiome, but not the individual diversity at the local scale (α-diversity) (Andermann et al. 2022). Arumugam et al. (2011) found that the prominent enterotypes of the human microbiome are the genera Bacteroides, Ruminococcus, and Prevotella. Different diets alter the distribution of these enterotypes (Wu et al. 2011), leading to an increase in Prevotella, Roseburia, Haemophilus, Neisseria, Aggregatibacter, and Veillonella species, which is associated with long-term adherence to a plant-based diet, although the Ruminococcus enterotype is conserved (David et al. 2014; De Filippo et al. 2010; Tomova et al. 2019; Zhang et al. 2018a). More recently, Xiao et al. (2022) reported that vegetarians had increased gut microbiota diversity compared to omnivores, with a higher abundance of the genera Prevotella, Clostridium, Lactobacillus, Ruminococcus, Eubacterium, and Faecalibacterium, and with lower loads of Bacteroides and Bifidobacterium.
VD has been considered as a healthy and therapeutic dietary pattern for several metabolic and chronic diseases, since it induces a reduction of pathobionts in the intestinal tract (Glick-Bauer and Yeh 2014). Besides, these diets are, in ecological terms, more sustainable than those that include meat, so their negative impact on the planet is significantly smaller (Craig et al. 2021).
The ketogenic diet (KD) is another emergent dietary habit, consisting in a normocaloric, high-fat, and very low-carbohydrate diet, which induces a state of ketosis (Attaye et al. 2021). KD feeding results in increased amounts of acetoacetate, β-hydroxybutyrate, and acetone in the blood and the urine, whose consequences are inhibition of apoptotic proteins, improvement of mitochondrial activity, attenuation of oxidative stress, expression of antioxidant proteins, and modulation of neurotransmitter levels (γ-aminobutyric acid [GABA], glutamate, and monoamines) (Cavaleri and Bashar 2018; Greco et al. 2016; Hartman et al. 2007; Yudkoff et al. 2008). These KD effects provide health benefits by reducing symptoms of affections such as autism, depression, epilepsy, diabetes, and various mental diseases (Bolla et al. 2019; Bostock et al. 2017; Lange et al. 2017; Martin-McGill et al. 2020).
Recent studies have suggested a key role for the gut microbiota in the mechanism of action of the KD (Attaye et al. 2021; Paoli et al. 2019; Rew et al. 2022). Kim et al. (2012) showed, in preclinical studies, that KD can increase inflammation and pro-inflammatory cytokines via the TLR4 signaling pathway. These changes are reflected in the composition of the gut microbiota, with a decrease in members of the Bacillota and an increase in members of the Bacteroidota phyla (Basciani et al. 2020). A study performed on humans, using a modified KD, found a decrease in the abundance of Bifidobacterium spp. and Lachnobacterium spp. in the gut microbiota; conversely, Akkermansia spp., Slackia spp., and members of the Christensenellaceae family were increased (Nagpal et al. 2019b). In children with severe epilepsy, Lindefeldt et al. (2019) did not found a significant change in the α-diversity of their fecal microbiota. However, a relative abundance of Bifidobacterium, Eubacterium, and Dialister were significantly diminished during the intervention, and an increase in relative abundance of Escherichia was also observed in KD-fed children. Another study, in animal and human models, showed that KD feeding decreased the abundance of members of Actinomycetota, Lactobacillus spp., and Bifidobacterium spp. (Ang et al. 2020). Additionally, these authors concluded that KD could potentially be used as a therapeutic tool to control autoimmune diseases, due to its reduction of pro-inflammatory Th17 cells. Nevertheless, a negative effect of the low carbohydrate diets, like KD, is the reduction in the abundance of the Bifidobacteria group, which has been positively associated with human health (Arboleya et al. 2016).
Effects of diet on neuropsychiatric and psychological disorders
Food is a source of several bioactive molecules, such as serotonin, dopamine, histamine, GABA, glutamate, and acetylcholine, which have neuroactive properties modulating neural signaling within the ENS, and influencing several brain functions (Briguglio et al. 2018; Burokas et al. 2015; Oriach et al. 2016). Omega-3 fatty acids and various amino acids may influence brain development and function independently of the gut microbiome (Sarris et al. 2015; Wani et al. 2015). Other dietary micronutrients, such as vitamins and minerals, can act as cofactors for enzymes, and be involved in neurotransmitter synthesis, myelination, cellular signaling, and metabolic pathways (Tardy et al. 2020).
Western diet
WD and obesity are risk factors for neuropsychiatric and psychological disorders, such as mild cognitive impairment, dementia, and depression (Castanon et al. 2014; Pedditzi et al. 2016; Pistell et al. 2010; Xu et al. 2011). WD components could induce neurochemical changes in specific brain regions; in particular, the dysfunction of the hippocampus leads to an impaired cognitive state, disrupting normal intake control and memory tasks (Francis and Stevenson 2013; Kanoski and Davidson 2011; Stevenson et al. 2020).
Long-term exposure to WD could produce addictive eating behaviors through the release of dopamine in the mesocorticolimbic system (Stevenson et al. 2020), and also the dysregulation of the HPA axis through the release of corticosterone, which provokes chronic stress, anxiety, and depression (López-Taboada et al. 2020; Makhathini et al. 2017; Thanarajah et al. 2019). On the other hand, stress triggers changes in the HPA axis that stimulate the production of leptin, ghrelin, insulin, or neuropeptide Y, establishing neuropeptide circuits that regulate intake control, memory, and motivation (Maniam and Morris 2012; Zanchi et al. 2017).
High-fat diets increase the proliferation of adipose tissue phagocytic cells, which leads to the release of pro-inflammatory cytokines and causes a neuroinflammation that is associated with depression, anxiety, and impaired cognitive function (Guillemot-Legris and Muccioli 2017; Kim et al. 2020; Seong et al. 2019). The high levels of endotoxins and the reduced levels of anti-inflammatory gut bacterial species are also linked to neuroinflammation (Noble et al. 2017).
Mediterranean diet
Numerous studies have shown that adherence to the MD is associated with a better cognition and memory (Lehert et al. 2015), a lower risk of cognitive impairment (Psaltopoulou et al. 2013; Singh et al. 2014; Wu and Sun 2017), a delay in cognitive decline (Chen et al. 2019; Morris et al. 2015), and in the development of neurodegenerative diseases (Karstens et al. 2019; Singh et al. 2014; van den Brink et al. 2019). These beneficial effects of this diet are due to its anti-inflammatory effects and to the increase of SCFAs (Koelman et al. 2022; Koh et al. 2016).
Several authors have pointed out that MD can reduce the risk of depression (Estruch et al. 2018; Huang et al. 2019; Jacka et al. 2017; Lassale et al. 2019), probably due to the abundance of oleic acid, polyphenols, and unsaturated fatty acids that are present in the dietary composition (Bayes et al. 2020; Del Chierico et al. 2014). Although a direct link between polyphenols and an improvement in neurological disorders has not yet been established, Wang et al. (2022) suggest that polyphenols may reverse neurodegenerative and neurocognitive pathologies. In addition, some studies have suggested a lower perceived stress and an improved stress resilience that is associated with the adherence to a MD (Bonaccio et al. 2018). The benefits of MD to improve the development of human health–related quality of life have been confirmed by different interventions in several countries, such as Italy (Bonaccio et al. 2013), Spain (Cabrera-Suárez et al. 2023; Galilea-Zabalza et al. 2018; Martínez-Lapiscina et al. 2013; Valls-Pedret et al. 2015), Greece and the UK (Klonizakis et al. 2019), Greece (Trichopoulou et al. 2015), Australia (Jacka et al. 2017), and the USA (Bhushan et al. 2018; Gigic et al. 2018).
Vegetarian diet
There are conflicting results regarding the influence of the VD on the incidence of depression; while some studies have found a positive association (Dobersek et al. 2021; Fazelian et al. 2022; Forestell and Nezlek 2018; Hibbeln et al. 2018; Kohl et al. 2023; Lavallee et al. 2019; Li et al. 2019; Matta et al. 2018; Stokes et al. 2011), others concluded that the VDs are not linked to depression (Beezhold and Johnston 2012; Beezhold et al. 2010; Jin et al. 2021; Michalak et al. 2012; Northstone et al. 2018; Norwood et al. 2019; Storz and Ronco 2023; Timko et al. 2012). It has been suggested that deficiencies of essential amino acids, such as methionine, tryptophan, and tyrosine, which are present at very low concentration in plant-based diets, may explain the relationship between these diets and the processes of depression (Schmidt et al. 2016), based on the metabolism of dopamine and serotonin (Aucoin et al. 2018).
Ketogenic diet
A large body of literature has reported that KD significantly reduces epileptic seizures, suggesting it as a therapeutic tool (Kossoff et al. 2018; Olson et al. 2018; Xie et al. 2017; Zhang et al. 2018b). KD helps to rebalance neurotransmitter systems, stabilizes neural networks, and improves neuroplasticity (Brietzke et al. 2018; Mujica-Parodi et al. 2020). Moreover, Campbell and Campbell (2020) suggested that KD may help to reduce the symptoms of bipolar disorder by bypassing the reduction of the mitochondrial defects.
The KD can also potentially cause cognitive impairment and alter the gut microbiota (increase of Bilophila wadsworthia). These facts disrupt hippocampal synaptic plasticity, neurogenesis, and gene expression (Olson et al. 2021). Shegelman et al. (2021), studying the effects of a KD in adult patients with chronic epilepsy, reported that this diet may have a beneficial effect on mental condition, reducing anxiety and depression. In a retrospective study, Danan et al. (2022) showed significant improvements in depression and in psychotic symptoms in individuals with severe mental disorders subjected to a KD. Similarly, Adams et al. (2022) reported an important enhancement in the mood of outpatients with type 2 diabetes that were treated with a KD for 2 years. However, Iacovides et al. (2019) found no significant differences in mood, cognitive performance, or subjective sleep quality, between the two groups of individuals (KD subjects and isocaloric high-carbohydrate low-fat diet subjects).
Diet, microbiome, and mental disorders
Dietary types change the composition and structure of the gut microbiome since birth with the introduction of solid foods in feeding, and with the aging (Claesson et al. 2012; Laursen et al. 2017). The gut microbiome of vaginally delivered infants is similar to the vaginal microbiota of the mother, while infants delivered by C-section acquired microbial communities found on human skin and opportunistic pathogens commonly associated with the healthcare environment (Dominguez-Bello et al. 2010) (Table 1). Breastfeeding provides a mixture of (i) nutrients that induce bacterial growth; (ii) antimicrobial agents; (iii) secreted-IgA that promotes a regulatory immune system; and (iv) human milk oligosaccharides, considered as prebiotics, that promotes the growth and function of beneficial microorganisms (O'Sullivan et al. 2015). The method of infant feeding by breastfeeding in the first 3 months of life shows a change in the microbial composition of the gut microbiome, with an increase in the abundance of the genera Bifidobacterium, Corynebacterium, Enterococcus, Lactobacillus, Propionibacterium, Sneathia, and Streptococcus (Table 1), and a decrease in the abundance of the genera Bacteroides and Staphylococcus. However, formula-fed infants have a different microbial composition, with the most prevalent bacterial genera being Atopobium (phylum Actinomycetota), Clostridium, Enterococcus, Lactobacillus, and Granulicatella (phylum Bacillota), Bacteroides (phylum Bacteroidota), Citrobacter, Enterobacter, and Escherichia (phylum Pseudomonadota), and Bilophila (phylum Thermodesulfobacteriota) (Cortes-Macías et al. 2021; Yao et al. 2021). During weaning, a variety of solid foods and new nutrients are introduced, and the microbial α-diversity and pH of the gut microbiome increase, resulting in the replacement of Actinomycetota and Pseudomonadota by Bacillota and Bacteroidota phyla as the dominant members of the infant gut microbiome (Koenig et al. 2011). In addition, between 9 and 18 months of age, a replacement of the dominant bacterial genera occur, and a difference was found in the gut microbiome of infants who were no longer breastfed compared to those who were breastfed longer; in the former, the most predominant genera are Akkermansia, Bacteroides, Bifidobacterium, Bilophila, and the members of the phylum Bacillota: Anaerostipes, Blautia, Clostridium, Faecalibacterium, Roseburia, and Ruminococcus; whereas in the longer breastfed the most abundant bacterial genera are Collinsella, and the members of the Bacillota phylum: Lactobacillus, Megasphaera, and Veillonella (Bäckhed et al. 2015; Vallès et al. 2014). These microbial changes are associated with the increased protein intake (members of Lachnospiraceae), dietary fiber intake (members of Prevotellacea), and increase in mucin production (genus Akkermansia) (Milani et al. 2017). The transition from an exclusively milk-based diet to solid foods induces the development of a mature microbiota containing genes responsible for degradation of complex carbohydrate and xenobiotic compounds, as well as those of vitamin production (Koenig et al. 2011). Thereafter, the microbiota remains unstable, suffering sudden microbial succession phenomena, until the infant is 2 to 3 years old, when the microbiota reaches a composition similar to that of adults (Yatsunenko et al. 2012).
These strong shifts in the gut microbiota profile have their consequences on several mental functions (David et al. 2014; Yatsunenko et al. 2012), through the secretion of biologically active compounds, including SCFAs, tryptophan catabolites, polyamines, and histamine (Lach et al. 2018; Lukić et al. 2022; Silva et al. 2020; Sudo 2019). The most quantitatively important metabolites are SCFAs that are produced by the microbial degradation of indigestible dietary fibers, proteins, and glycoproteins (Wong et al. 2006). SCFAs, such as butyrate, acetate, and propionate, can act as signaling molecules that locally modulate the gut function from the duodenum to the colon; and through enteroendocrine cells they can also control liver, muscle, and brain metabolism, thereby influencing the host energy homeostasis (Dalile et al. 2019; den Besten et al. 2013; Silva et al. 2020). In addition, SCFAs have neuroactive properties through the induction of neuroinflammatory responses (Dalile et al. 2019). Other biologically active compounds are indole and 5-hydroxytryptamine, derived from tryptophan metabolism, which regulate the secretion of the serotonin and melatonin (Lukić et al. 2022), and may serve as signaling molecules for intercellular communication between microorganisms and host cells (Lee and Lee 2010).
Tables 2 and 3 show the relationship between diet, the shape of the gut microbiome, its metabolomes, and mental disorders. WD induces a shift in the gut microbiome composition, which induces the synthesis of microbial metabolites and soluble substances that affect brain functions through the gut microbiota–brain axis, increasing the symptoms of schizophrenia and dementia, as well as the effects on several psychological features, such as cognitive impairment, stress, depression, and anxiety (Aucoin et al. 2018; Jacka et al. 2017; López-Taboada et al. 2020) (Table 2). On the contrary, MD promotes the production of bacterial intermediates that reduce anxiety, depression, and symptoms of bipolar disorder and schizophrenia, and also the increase of stress resilience (Bonaccio et al. 2013; Dinu et al. 2022; Madani et al. 2022) (Table 2).
Regarding VD, contradictory results were observed, since SCFAs, polyamines from protein metabolism, and retinoic acids, reduce anxiety, depression, cognitive impairment, and Alzheimer’s symptoms; whereas other intermediates, such as GABA, TMAO, LPS, and 5-hydroxytryptamine, increase affective disorders and depression (Iguacel et al. 2021; Jain et al. 2022) (Table 3). KD induces the synthesis of ketone bodies and suppresses the release of SCFAs and 5-hydroxytryptamine. These intermediates increase depression, but reduce epileptic seizure symptoms, amyloid plaque deposition, and autism symptoms (Grigolon et al. 2020; Shegelman et al. 2021; Tillery et al. 2021) (Table 3).
Future perspectives and conclusions
Diet is the most important environmental factor influencing the composition and shape of the gut microbiome. This microbiome plays a critical role in host health, including the development of the immune system, the metabolism of nutrients, and the synthesis of bioactive molecules. The mechanisms of action and the extent to which bacterial metabolites may influence the brain function are poorly understood, due to the complexity of the pathways involved in the gut–brain axis; however, this deficiency could be addressed with new advances in metabolomic technology. In this sense, new sequencing technologies will allow us to gain a deeper insight into the composition of the microbiota and its association with neuropsychiatric and psychological disorders.
In recent years, a growing body of research has been focused in the interplay between dietary patterns, gut microbiota composition, and their collective influence on mental health (Taylor et al. 2019). Thus, the role of diet in the etiology and treatment of neuropsychiatric disorders has been proposed (Sarris et al. 2015). Nevertheless, the influence of diet on brain health is a complex and multifactorial-dependent process. Therefore, how nutrition affects gut microbiota composition, and its consequences in psychiatric and psychological disorders, need to be clarified in future by the improvement of human clinical interventions. Further research in this area, then, will elucidate the modulating effects of dietary components on the intestinal microbiota, as well as their potential impact on psychological well-being. Although different types of diets have already been studied with that purpose, it could be pertinent to assess the divergent impacts of dietary variations under the general diet categorization. In this regard, especially the variants of plant-based diets should not be examined together, given the significant difference between them with respect to the presence of certain foods.
Two types of intervention, probiotic and dietary, have been proposed to link gut microbiota, behavioral changes, and diet. Several psychobiotics added as dietary supplements may improve brain functioning and act as therapeutic tools for neuropsychiatric disorders (Dinan et al. 2013). Prebiotic and dietary interventions, including high-fat foods and polyphenols, may be feasible as long-term interventions to reduce certain mental disorders. The use of aliments with a high content of polyamines, as well as the use of probiotics that influence gut bacteria to synthesize polyamines, could be promising approaches for the prevention of cognitive impairment, including Alzheimer’s disease (Hirano et al. 2021; Valdes et al. 2018; Wang et al. 2022). In this regard, new strategies would be based on the development of probiotic treatment with newer bacteria, on the combination of probiotics and prebiotics (synbiotics), and on personalized nutritional interventions (Chua et al. 2017; Zamroziewicz and Barbey 2016; Zeevi et al. 2015). The development of predictive nutrient patterns will provide empirical nutrition therapies for the treatment of cognitive and neurological impairments in the brain.
Therefore, future methodological advances in nutritional epidemiology are needed, through the development of biochemical markers of dietary intake, studies of holistic dietary patterns, and the application of nutrient biomarker patterns (NBP). The use of the NBP method has revealed various nutrient patterns that influence cognitive impairment and brain disorders (Bowman et al. 2012). Metabolomics could provide a tool to investigate the interactions between dietary components and mental function, by characterizing individual dietary phenotypes and elucidating the mechanisms of health status. However, the validation of dietary markers, and the identification of specific patterns of the food metabolome associated with a healthy brain, are still unknown (Scalbert et al. 2014).
Nutritional psychiatry is a relatively new field of research that has evolved from preclinical outcomes and a series of cross-sectional and epidemiological studies linking diet to various aspects of mental health, as well as from the insights of microbiome science, which have provided a relationship between diet, microbial function, and brain health (Adan et al. 2019; Teasdale et al. 2020). The potential future implications of nutritional psychiatry will include the role of diagnostic testing of the gut microbiome to identify targets for personalized psychological and psychiatric treatments, and the potential for integrative approaches combining dietary interventions, pharmacotherapy, and cognitive-behavioral treatments.
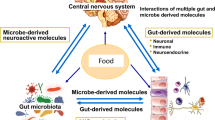
In conclusion, the gut microbiome-brain network connects three compartments: the brain, the gut, and the microbiome. All of them are linked by bidirectional connections with multiple feedback loops, creating a non-linear system. Different dietary components influence the brain, the gut, and the gut microbiome through different communication channels. Dietary components can directly affect the gut and reach the brain after absorption in the small intestine. Diet can also influence the composition and diversity of the gut microbiota and the microbial metabolism, thereby modulating the gut connectome. Some of the microbial-derived molecules are absorbed and reach the brain via the systemic circulation and/or the vagus nerve. Furthermore, the brain can modulate the microbiome directly through the action of neuroactive substances released into the gut lumen that affect microbial gene expression, or indirectly by altering the environment of the gut microbiome. On the other hand, there is a need for clinical evidence, based on randomized controlled trials, and prebiotics, probiotics or fecal microbiota transplantation, to assess dietary changes in gut microbiota composition and their influence on psychological outcomes.
Data availability
No datasets were generated or analysed during the current study.
References
Adams JB, Audhya T, Geis E, Gehn E, Fimbres V, Pollard EL et al (2018) Comprehensive nutritional and dietary intervention for autism spectrum disorder - a randomized, controlled 12-month trial. Nutrients 10:369. https://doi.org/10.3390/nu10030369
Adams RN, Athinarayanan SJ, McKenzie AL, Hallberg SJ, McCarter JP, Phinney SD et al (2022) Depressive symptoms improve over 2 years of type 2 diabetes treatment via a digital continuous remote care intervention focused on carbohydrate restriction. J Behav Med 45:416–427. https://doi.org/10.1007/s10865-021-00272-4
Adan RAH, van der Beek EM, Buitelaar JK, Cryan JF, Hebebrand J, Higgs S et al (2019) Nutritional psychiatry: towards improving mental health by what you eat. Eur Neuropsychopharmacol 29:1321–1332. https://doi.org/10.1016/j.euroneuro.2019.10.011
Almonacid DE, Kraal L, Ossandon FJ, Budovskaya YV, Cardenas JP et al (2019) Correction: 16S rRNA gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome. PLoS One 14:e0212474. https://doi.org/10.1371/journal.pone.0212474
Andermann T, Antonelli A, Barrett RL, Silvestro D (2022) Estimating alpha, beta, and gamma diversity through deep learning. Front Plant Sci 13:839407. https://doi.org/10.3389/fpls.2022.839407
Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V et al (2020) Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 181:1263-1275.e16. https://doi.org/10.1016/j.cell.2020.04.027
Arboleya S, Watkins C, Stanton C, Ross RP (2016) Gut bifidobacteria populations in human health and aging. Front Microbiol 7:1204. https://doi.org/10.3389/fmicb.2016.01204
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR et al (2011) Enterotypes of the human gut microbiome. Nature 473:174–180. https://doi.org/10.1038/nature09944
Attaye I, van Oppenraaij S, Warmbrunn MV, Nieuwdorp M (2021) The role of the gut microbiota on the beneficial effects of ketogenic diets. Nutrients 14:191. https://doi.org/10.3390/nu14010191
Aucoin M, LaChance L, Cooley K, Kidd S (2018) Diet and psychosis: a scoping review. Neuropsychobiology 79:20–42. https://doi.org/10.1159/000493399
Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P et al (2015) Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17:690–703. https://doi.org/10.1016/j.chom.2015.04.004
Basciani S, Camajani E, Contini S, Persichetti A, Risi R, Bertoldi L et al (2020) Very-low-calorie ketogenic diets with whey, vegetable, or animal protein in patients with obesity: a randomized pilot study. J Clin Endocrinol Metab 105:2939–2949. https://doi.org/10.1210/clinem/dgaa336
Bayes J, Schloss J, Sibbritt D (2020) Effects of polyphenols in a Mediterranean diet on symptoms of depression: a systematic literature review. Adv Nutr 11:602–615. https://doi.org/10.1093/advances/nmz117
Beam A, Clinger E, Hao L (2021) Effect of diet and dietary components on the composition of the gut microbiota. Nutrients 13:2795. https://doi.org/10.3390/nu13082795
Beezhold BL, Johnston CS (2012) Restriction of meat, fish, and poultry in omnivores improves mood: a pilot randomized controlled trial. Nutr J 11:9. https://doi.org/10.1186/1475-2891-11-9
Beezhold B, Johnston C, Daigle D (2010) Vegetarian diets are associated with healthy mood states: a cross-sectional study in Seventh Day Adventist adults. Nutr J 9:1–7. https://doi.org/10.1186/1475-2891-9-26
Berg G, Rybakova D, Fischer D, Cernava T, Vergès MC, Charles T et al (2020) Microbiome definition re-visited: old concepts and new challenges. Microbiome 8:103. https://doi.org/10.1186/s40168-020-00875-0
Bhushan A, Fondell E, Ascherio A, Yuan C, Grodstein F, Willett W (2018) Adherence to Mediterranean diet and subjective cognitive function in men. Eur J Epidemiol 33:223–234. https://doi.org/10.1007/s10654-017-0330-3
Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ (2019) Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe 26:265-272.e4. https://doi.org/10.1016/j.chom.2019.06.013
Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L (2019) Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients 11:962. https://doi.org/10.3390/nu11050962
Bonaccio M, Di Castelnuovo A, Bonanni A, Costanzo S, De Lucia F, Pounis G et al (2013) Adherence to a Mediterranean diet is associated with a better health-related quality of life: a possible role of high dietary antioxidant content. BMJ Open 3:e003003. https://doi.org/10.1136/bmjopen-2013-003003
Bonaccio M, Di Castelnuovo A, Costanzo S, Pounis G, Persichillo M, Cerletti C et al (2018) Mediterranean-type diet is associated with higher psychological resilience in a general adult population: findings from the Moli-sani study. Eur J Clin Nutr 72:154–160. https://doi.org/10.1038/ejcn.2017.150
Borrego-Ruiz A, Borrego JJ (2024) An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog Neuropsychopharmacol Biol Psychiatry 128:118061. https://doi.org/10.1016/j.pnpbp.2023.110861
Bostock EC, Kirkby KC, Taylor BV (2017) The current status of the ketogenic diet in Psychiatry. Front Psychiatry 8:43. https://doi.org/10.3389/fpsyt.2017.00043
Bowman GL, Silbert LC, Howieson D, Dodge HH, Traber MG, Frei B et al (2012) Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology 78:241–249. https://doi.org/10.1212/WNL.0b013e3182436598
Brietzke E, Mansur RB, Subramaniapillai M, Balanzá-Martínez V, Vinberg M, González-Pinto A et al (2018) Ketogenic diet as a metabolic therapy for mood disorders: evidence and developments. Neurosci Biobehav Rev 94:11–16. https://doi.org/10.1016/j.neubiorev.2018.07.020
Briguglio M, Dell’Osso B, Panzica G, Malgaroli A, Banfi G, Zanaboni Dina C et al (2018) Dietary neurotransmitters: a narrative review on current knowledge. Nutrients 10:591. https://doi.org/10.3390/nu10050591
Burokas A, Moloney RD, Dinan TG, Cryan JF (2015) Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol 91:1–62. https://doi.org/10.1016/bs.aambs.2015.02.001
Cabrera-Suárez BM, Lahortiga-Ramos F, Sayon-Orea C, Hernández-Fleta JL, González-Pinto A, Molero P et al (2023) Effect of a dietary intervention based on the Mediterranean diet on the quality of life of patients recovered from depression: analysis of the PREDIDEP randomized trial. Exp Gerontol 175:12149, 1. https://doi.org/10.1016/j.exger.2023.112149
Campbell I, Campbell H (2020) Mechanisms of insulin resistance, mitochondrial dysfunction and the action of the ketogenic diet in bipolar disorder. Focus on the PI3K/AKT/HIF1-a pathway. Med Hypotheses 145:110299. https://doi.org/10.1016/j.mehy.2020.110299
Cani PD (2013) Gut microbiota and obesity: lessons from the microbiome. Brief Funct Genomics 12:381–387. https://doi.org/10.1093/bfgp/elt014
Cantarel BL, Lombard V, Henrissat B (2012) Complex carbohydrate utilization by the healthy human microbiome. PLoS One 7:e28742. https://doi.org/10.1371/journal.pone.0028742
Castanon N, Lasselin J, Capuron L (2014) Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol 5:74. https://doi.org/10.3389/fendo.2014.00074
Cavaleri F, Bashar E (2018) Potential synergies of β-hydroxybutyrate and butyrate on the modulation of metabolism, inflammation, cognition, and general health. J Nutr Metab 2018:7195760. https://doi.org/10.1155/2018/7195760
Chen X, Maguire B, Brodaty H, O’Leary F (2019) Dietary patterns and cognitive health in older adults: a systematic review. J Alzheimers Dis 67:583–619. https://doi.org/10.3233/JAD-180468
Chua KJ, Kwok WC, Aggarwal N, Sun T, Chang MW (2017) Designer probiotics for the prevention and treatment of human diseases. Curr Opin Chem Biol 40:8–16. https://doi.org/10.1016/j.cbpa.2017.04.011
Claesson M, Jeffery I, Conde S, Power SE, O’Connor EM, Cusack S et al (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488:178–184. https://doi.org/10.1038/nature11319
Clemente-Suárez VJ, Beltrán-Velasco AI, Redondo-Flórez L, Martín-Rodríguez A, Tornero-Aguilera JF (2023) Global impacts of Western diet and its effects on metabolism and health: a narrative review. Nutrients 15:2749. https://doi.org/10.3390/nu15122749
Cortes-Macías E, Selma-Royo M, García-Mantrana I, Calatayud M, González S, Martínez-Costa C et al (2021) Maternal diet shapes the breast milk microbiota composition and diversity: impact of mode of delivery and antibiotic exposure. J Nutr 151:330–340. https://doi.org/10.1093/jn/nxaa310
Costea PI, Hildebrand F, Arumugam M, Bäckhed F, Blaser MJ, Bushman FD et al (2018) Enterotypes in the landscape of gut microbial community composition. Nat Microbiol 3:8–16. https://doi.org/10.1038/s41564-017-0072-8
Craig WJ, Mangels AR, Fresán U, Marsh K, Miles FL, Saunders AV et al (2021) The safe and effective use of plant-based diets with guidelines for health professionals. Nutrients 13:4144. https://doi.org/10.3390/nu13114144
Cryan JF, Dinan TG (2015) More than a gut feeling: the microbiota regulates neurodevelopment and behavior. Neuropsychopharmacology 40:241–242. https://doi.org/10.1038/npp.2014.224
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M et al (2019) The microbiota-gut-brain axis. Physiol Rev 99:1877–2013. https://doi.org/10.1152/physrev.00018.2018
Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG (2020) The gut microbiome in neurological disorders. Lancet Neurol 19:179–194. https://doi.org/10.1016/S1474-4422(19)30356-4
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K (2019) The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 16:461–478. https://doi.org/10.1038/s41575-019-0157-3
Danan A, Westman EC, Saslow LR, Ede G (2022) The ketogenic diet for refractory mental illness: a retrospective analysis of 31 inpatients. Front Psychiatry 13:951376. https://doi.org/10.3389/fpsyt.2022.951376
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. https://doi.org/10.1038/nature12820
De Filippis F, Pellegrini N, Vannini L, Jeffery I, La Storia A, Laghi L et al (2016) High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65:1812–1821. https://doi.org/10.1136/gutjnl-2015-309957
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S et al (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107:14691–14696. https://doi.org/10.1073/pnas.1005963107
Del Chierico F, Vernocchi P, Dallapiccola B, Putignani L (2014) Mediterranean diet and health: food effects on gut microbiota and disease control. Int J Mol Sci 15:11678–11699. https://doi.org/10.3390/ijms150711678
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. https://doi.org/10.1194/jlr.R036012
Dinan TG, Stanton C, Cryan JF (2013) Psychobiotics: a novel class of psychotropic. Biol Psychiatry 74:720–726. https://doi.org/10.1016/j.biopsych.2013.05.001
Dinu M, Lotti S, Napoletano A, Corrao A, Pagliai G, Tristan Asensi M et al (2022) Association between psychological disorders, Mediterranean diet, and chronotype in a group of Italian adults. Int J Environ Res Public Health 20:335. https://doi.org/10.3390/ijerph20010335
Dobersek U, Wy G, Adkins J, Altmeyer S, Krout K, Lavie CJ et al (2021) Meat and mental health: a systematic review of meat abstention and depression, anxiety, and related phenomena. Crit Rev Food Sci Nutr 61:622–635. https://doi.org/10.1080/10408398.2020.1741505
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 107:11971–11975. https://doi.org/10.1073/pnas.1002601107
Eilam O, Zarecki R, Oberhardt M, Ursell LK, Kupiec M, Knight R et al (2014) Glycan degradation (GlyDeR) analysis predicts mammalian gut microbiota abundance and host diet-specific adaptations mBio 5:e01526–14. https://doi.org/10.1128/mBio.01526-14
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F et al (2018) Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 378:e34. https://doi.org/10.1056/NEJMoa1800389
Fazelian S, Sadeghi E, Firouzi S, Haghighatdoost F (2022) Adherence to the vegetarian diet may increase the risk of depression: a systematic review and meta-analysis of observational studies. Nutr Rev 80:242–254. https://doi.org/10.1093/nutrit/nuab013
Forestell CA, Nezlek JB (2018) Vegetarianism, depression, and the five factor model of personality. Ecol Food Nutr 57:246–259. https://doi.org/10.1080/03670244.2018.1455675
Francis H, Stevenson R (2013) The longer-term impacts of Western diet on human cognition and the brain. Appetite 63:119–128. https://doi.org/10.1016/j.appet.2012.12.018
Galilea-Zabalza I, Buil-Cosiales P, Salas-Salvadó J, Toledo E, Ortega-Azorín C, Díez-Espino J et al (2018) Mediterranean diet and quality of life: baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS One 13:e0198974. https://doi.org/10.1371/journal.pone.0198974eCollection2018
Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC (2018) Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol 9:890. https://doi.org/10.3389/fmicb.2018.00890
Gerber GK (2014) The dynamic microbiome. FEBS Lett 588:4131–4139. https://doi.org/10.1016/j.febslet.2014.02.037
Ghosh T, Rampelli S, Jeffery IB, Santoro A, Neto MC, Capri M et al (2020) Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 69:1218–1228. https://doi.org/10.1136/gutjnl-2019-319654
Gigic B, Boeing H, Toth R, Böhm J, Habermann N, Scherer D et al (2018) Associations between dietary patterns and longitudinal quality of life changes in colorectal cancer patients: the ColoCare study. Nutr Cancer 70:51–60. https://doi.org/10.1080/01635581.2018.1397707
Gilbert JA, Lynch SV (2019) Community ecology as a framework for human microbiome research. Nat Med 25:884–889. https://doi.org/10.1038/s41591-019-0464-9
Glick-Bauer M, Yeh MC (2014) The health advantage of a vegan diet: exploring the gut microbiota connection. Nutrients 6:4822–4838. https://doi.org/10.3390/nu6114822
Greco T, Glenn TC, Hovda DA, Prins ML (2016) Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cereb Blood Flow Metab 36:1603–1613. https://doi.org/10.1177/0271678X15610584
Grigolon RB, Gerchman F, Schöffel AC, Hawken ER, Gill H, Vazquez GH et al (2020) Mental, emotional, and behavioral effects of ketogenic diet for non-epileptic neuropsychiatric conditions. Prog Neuropsychopharmacol Biol Psychiatry 102:109947. https://doi.org/10.1016/j.pnpbp.2020.109947
Guasch-Ferré M, Willett WC (2021) The Mediterranean diet and health: a comprehensive overview. J Intern Med 290:549–566. https://doi.org/10.1111/joim.13333
Guillemot-Legris O, Muccioli GG (2017) Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci 40:237–253. https://doi.org/10.1016/j.tins.2017.02.005
Hartman AL, Gasior M, Vining EP, Rogawski MA (2007) The neuropharmacology of the ketogenic diet. Pediatr Neurol 36:281–292. https://doi.org/10.1016/j.pediatrneurol.2007.02.008
Haro C, García-Carpintero S, Rangel-Zúñiga OA, Alcalá-Díaz JF, Landa BB, Clemente JC et al (2017) Consumption of two healthy dietary patterns restored microbiota dysbiosis in obese patients with metabolic dysfunction. Mol Nutr Food Res 61:1700300. https://doi.org/10.1002/mnfr.201700300
Hargreaves SM, Rosenfeld DL, Moreira AVB, Zandonadi RP (2023) Plant-based and vegetarian diets: an overview and definition of these dietary patterns. Eur J Nutr 62:1109–1121. https://doi.org/10.1007/s00394-023-03086-z
Herpich C, Müller-Werdan U, Norman K (2022) Role of plant-based diets in promoting health and longevity. Maturitas 165:47–51. https://doi.org/10.1016/j.maturitas.2022.07.003
Hibbeln JR, Northstone K, Evans J, Golding J (2018) Vegetarian diets and depressive symptoms among men. J Affect Disord 225:13–17. https://doi.org/10.1016/j.jad.2017.07.051
Hibberd MC, Wu M, Rodionov DA, Li X, Cheng J, Griffin NW et al (2017) The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci Transl Med 9:eaal4069. https://doi.org/10.1126/scitranslmed.aal4069
Hirano R, Shirasawa H, Kurihara S (2021) Health-promoting effects of dietary polyamines. Med Sci 9:8. https://doi.org/10.3390/medsci9010008
Horn J, Mayer DE, Chen S, Mayer EA (2022) Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl Psychiatry 12:164. https://doi.org/10.1038/s41398-022-01922-0
Huang Q, Liu H, Suzuki K, Ma S, Liu C (2019) Linking what we eat to our mood: A review of diet, dietary antioxidants, and depression. Antioxidants 8:376. https://doi.org/10.3390/antiox8090376
Iacovides S, Goble D, Paterson B, Meiring RM (2019) Three consecutive weeks of nutritional ketosis has no effect on cognitive function, sleep, and mood compared with a high-carbohydrate, low-fat diet in healthy individuals: a randomized, crossover, controlled trial. Am J Clin Nutr 110:349–357. https://doi.org/10.1093/ajcn/nqz073
Iguacel I, Huybrechts I, Moreno LA, Michels N (2021) Vegetarianism and veganism compared with mental health and cognitive outcomes: a systematic review and meta-analysis. Nutr Rev 79:361–381. https://doi.org/10.1093/nutrit/nuaa030
Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M et al (2017) A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med 15:23. https://doi.org/10.1186/s12916-017-0791-y
Jain R, Larsuphrom P, Degremont A, Latunde-Dada GO, Philippou E (2022) Association between vegetarian and vegan diets and depression: a systematic review. Nutr Bull 47:27–49. https://doi.org/10.1111/nbu.12540
Jin Y, Kandula NR, Kanaya AM, Talegawkar SA (2021) Vegetarian diet is inversely associated with prevalence of depression in middle-older aged South Asians in the United States. Ethn Health 26:504–511. https://doi.org/10.1080/13557858.2019.1606166
Kanoski SE, Davidson TL (2011) Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav 103:59–68. https://doi.org/10.1016/j.physbeh.2010.12.003
Karstens AJ, Tussing-Humphreys L, Zhan L, Rajendran N, Cohen J, Dion C et al (2019) Associations of the Mediterranean diet with cognitive and neuroimaging phenotypes of dementia in healthy older adults. Am J Clin Nutr 109:361–368. https://doi.org/10.1093/ajcn/nqy275
Kim KA, Gu W, Lee IA, Joh EH, Kim DH (2012) High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7:e47713. https://doi.org/10.1371/journal.pone.0047713
Kim MH, Yun KE, Kim J, Park E, Chang Y, Ryu S et al (2020) Gut microbiota and metabolic health among overweight and obese individuals. Sci Rep 10:19417. https://doi.org/10.1038/s41598-020-76474-8
Klonizakis M, Grammatikopoulou MG, Theodoridis X, Milner M, Liu Y, Chourdakis M (2019) Effects of long-versus short-term exposure to the Mediterranean diet on skin microvascular function and quality of life of healthy adults in Greece and the UK. Nutrients 11:2487. https://doi.org/10.3390/nu11102487
Koelman L, Rodrigues C, Aleksandrova K (2022) Effects of dietary patterns on biomarkers of inflammation and immune responses: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 13:101–115. https://doi.org/10.1093/advances/nmab086
Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R et al (2011) Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA 108:4578–4585. https://doi.org/10.1073/pnas.1000081107
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Kohl IS, Luft VC, Patrão AL, Molina MDCB, Nunes MAA, Schmidt MI (2023) Association between meatless diet and depressive episodes: a cross-sectional analysis of baseline data from the longitudinal study of adult health (ELSA-Brasil). J Affect Disord 320:48–56. https://doi.org/10.1016/j.jad.2022.09.059
Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R et al (2018) Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 3:175–192. https://doi.org/10.1002/epi4.12225
Lach G, Schellekens H, Dinan TG, Cryan JF (2018) Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15:36–59. https://doi.org/10.1007/s13311-017-0585-0
Lange KW, Lange KM, Makulska-Gertruda E, Nakamura Y, Reissmann A, Kanaya S et al (2017) Ketogenic diets and Alzheimer’s disease. Food Sci Hum Wellness 6:1–9. https://doi.org/10.3390/ijms20163892
Lassale C, Beulens J, Van der Schouw Y, Roswall N, Weiderpass E, Romaguera D et al (2015) A pro-vegetarian food pattern and cardiovascular mortality in the EPIC study. Circulation 131:A16. https://doi.org/10.1161/circ.131.suppl_1.16
Lassale C, Batty GD, Baghdadli A, Jacka F, Sánchez-Villegas A, Kivimäki M et al (2019) Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry 24:965–986. https://doi.org/10.1038/s41380-018-0237-8
Laursen M, Bahl MI, Michaelsen KF, Licht TR (2017) First foods and gut microbes. Front Microbiol 8:356. https://doi.org/10.3389/fmicb.2017.00356
Lavallee K, Zhang XC, Michalak J, Schneider S, Margraf J (2019) Vegetarian diet and mental health: cross-sectional and longitudinal analyses in culturally diverse samples. J Affect Disord 248:147–154. https://doi.org/10.1016/j.jad.2019.01.035
Lee JH, Lee J (2010) Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. https://doi.org/10.1111/j.1574-6976.2009.00204.x
Lehert P, Villaseca P, Hogervorst E, Maki PM, Henderson VW (2015) Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric 18:678–689. https://doi.org/10.3109/13697137.2015.1078106
Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S et al (2014) An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 32:834–841. https://doi.org/10.1038/nbt.2942
Li XD, Cao HJ, Xie SY, Li KC, Tao FB, Yang LS et al (2019) Adhering to a vegetarian diet may create a greater risk of depressive symptoms in the elderly male Chinese population. J Affect Disord 243:182–187. https://doi.org/10.1016/j.jad.2018.09.033
Lindefeldt M, Eng A, Darban H, Bjerkner A, Zetterström CK, Allander T et al (2019) The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 5:5. https://doi.org/10.1038/s41522-018-0073-2
Lopez-Legarrea P, Fuller NR, Zulet MA, Martinez JA, Caterson ID (2014) The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac J Clin Nutr 23:360–368. https://doi.org/10.6133/apjcn.2014.23.3.16
López-Taboada I, González-Pardo H, Conejo NM (2020) Western diet: implications for brain function and behavior. Front Psychol 11:564413. https://doi.org/10.3389/fpsyg.2020.564413
Lukić I, Ivković S, Mitić M, Adžić M (2022) Tryptophan metabolites in depression: modulation by gut microbiota. Front Behav Neurosci 16:987697. https://doi.org/10.3389/fnbeh.2022.987697
Luthold RV, Fernandes GR, Franco-de-Moraes AC, Folchetti LG, Ferreira SR (2017) Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metab Clin Exp 69:76–86. https://doi.org/10.1016/j.metabol.2017.01.007
Madani S, Ahmadi A, Shoaei-Jouneghani F, Moazen M, Sasani N (2022) The relationship between the Mediterranean diet and axis I disorders: a systematic review of observational studies. Food Sci Nutr 10:3241–3258. https://doi.org/10.1002/fsn3.2950
Makhathini KB, Abboussi O, Stein DJ, Mabandla MV, Daniels WMU (2017) Repetitive stress leads to impaired cognitive function that is associated with DNA hypomethylation, reduced BDNF and a dysregulated HPA axis. Int J Dev Neurosci 60:63–69. https://doi.org/10.1016/j.ijdevneu.2017.04.004
Malinowska AM, Kok DE, Steegenga WT, Hooiveld G, Chmurzynska A (2022) Human gut microbiota composition and its predicted functional properties in people with Western and healthy dietary patterns. Eur J Nutr 61:3887–3903. https://doi.org/10.1007/s00394-022-02928-6
Maniam J, Morris MJ (2012) The link between stress and feeding behaviour. Neuropharmacology 63:97–110. https://doi.org/10.1016/j.neuropharm.2012.04.017
Marlow G, Ellett S, Ferguson IR, Zhu S, Karunasinghe N, Jesuthasan AC et al (2013) Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum Genomics 7:24. https://doi.org/10.1186/1479-7364-7-24
Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN (2020) Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev 6:CD001903. https://doi.org/10.1002/14651858.CD001903.pub5
Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, San Julián B et al (2013) Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry 84:1318–1325. https://doi.org/10.1136/jnnp-2012-304792
Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K et al (2021) Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry 26:134–150. https://doi.org/10.1038/s41380-020-00925-x
Matta J, Czernichow S, Kesse-Guyot E, Hoertel N, Limosin F, Goldberg M et al (2018) Depressive symptoms and vegetarian diets: results from the Constances cohort. Nutrients 10:1695. https://doi.org/10.3390/nu10111695
McMacken M, Shah, S (2017) A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol 14:342–354. https://doi.org/10.11909/j.issn.1671-5411.2017.05.009
Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR et al (2017) Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology 152:1944-1953.e1. https://doi.org/10.1053/j.gastro.2017.02.015
Michalak J, Zhang XC, Jacobi F (2012) Vegetarian diet and mental disorders: results from a representative community survey. Int J Behav Nutr Phys Act 9:67. https://doi.org/10.1186/1479-5868-9-67
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J et al (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev 81:e00036-e117. https://doi.org/10.1128/MMBR.00036-17
Mitsou EK, Kakali A, Antonopoulou S, Mountzouris KC, Yannakoulia M, Panagiotakos DB et al (2017) Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr 117:1645–1655. https://doi.org/10.1017/S0007114517001593
Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA et al (2015) MIND diet slows cognitive decline with aging. Alzheimers Dement 11:1015–1022. https://doi.org/10.1016/j.jalz.2015.04.011
Mujica-Parodi LR, Amgalan A, Sultan SF, Antal B, Sun X, Skiena S et al (2020) Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc Natl Acad Sci USA 117:6170–6177. https://doi.org/10.1073/pnas.1913042117
Muscogiuri G, Cantone E, Cassarano S, Tuccinardi D, Barrea L, Savastano S et al (2019) Gut microbiota: a new path to treat obesity. Int J Obes Suppl 9:10–19. https://doi.org/10.1038/s41367-019-0011-7
Muscogiuri G, Verde L, Sulu C, Katsiki N, Hassapidou M, Frias-Toral E et al (2022) Mediterranean diet and obesity-related disorders: what is the evidence? Curr Obes Rep 11:287–304. https://doi.org/10.1007/s13679-022-00481-1
Nagpal R, Shively CA, Register TC, Craft S, Yadav H (2019a) Gut microbiome-Mediterranean diet interactions in improving host health. F1000Res 8:699. https://doi.org/10.12688/f1000research.18992.1
Nagpal R, Neth BJ, Wang S, Craft S, Yadav H (2019b) Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47:529–542. https://doi.org/10.1016/j.ebiom.2019.08.032
Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H et al (2016) The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res 23:125–133. https://doi.org/10.1093/dnares/dsw002
Noble EE, Hsu TM, Kanoski SE (2017) Gut to brain dysbiosis: mechanisms linking Western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci 11:9. https://doi.org/10.3389/fnbeh.2017.00009
Northstone K, Joinson C, Emmett P (2018) Dietary patterns and depressive symptoms in a UK cohort of men and women: a longitudinal study. Public Health Nutr 21:831–837. https://doi.org/10.1017/S1368980017002324
Norwood R, Cruwys T, Chachay VS, Sheffield J (2019) The psychological characteristics of people consuming vegetarian, vegan, paleo, gluten free and weight loss dietary patterns. Obes Sci Pract 5:148–158. https://doi.org/10.1002/osp4.325
Nuriel-Ohayon M, Neuman H, Koren O (2016) Microbial changes during pregnancy, birth, and infancy. Front Microbiol 7:1031. https://doi.org/10.3389/fmicb.2016.01031
Olfert MD, Barr ML, Mathews AE, Horacek TM, Riggsbee K, Zhou W et al (2022) Life of a vegetarian college student: health, lifestyle, and environmental perceptions. J Am Coll Health 70:232–239. https://doi.org/10.1080/07448481.2020.1740231
Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY (2018) The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173:1728-1741.e13. https://doi.org/10.1016/j.cell.2018.04.027
Olson CA, Iñiguez AJ, Yang GE, Fang P, Pronovost GN, Jameson KG et al (2021) Alterations in the gut microbiota contribute to cognitive impairment induced by the ketogenic diet and hypoxia. Cell Host Microb 29:1378-1392.e6. https://doi.org/10.1016/j.chom.2021.07.004
O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF (2015) Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277:32–48. https://doi.org/10.1016/j.bbr.2014.07.027
Oriach CS, Robertson RC, Stanton C, Cryan JF, Dinan TG (2016) Food for thought: the role of nutrition in the microbiota-gut-brain axis. Clin Nutr Exp 6:25–38. https://doi.org/10.1016/j.yclnex.2016.01.003
Orlich MJ, Singh PN, Sabaté J, Jaceldo-Siegl K, Fan J, Knutsen S et al (2013) Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med 173:1230–1238. https://doi.org/10.1001/jamainternmed.2013.6473
Orlich MJ, Singh PN, Sabaté J, Fan J, Sveen L, Bennett H et al (2015) Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med 175:767–776. https://doi.org/10.1001/jamainternmed.2015.59
O’Sullivan A, Farver M, Smilowitz JT (2015) The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr Metab Insights 8:1–9. https://doi.org/10.4137/NMI.S29530
Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H et al (2019) Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosc Lett 690:232–236. https://doi.org/10.1016/j.neulet.2018.10.048
Pagliai G, Russo E, Niccolai E, Dinu M, Di Pilato V, Magrini A et al (2020) Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: The CARDIVEG Study. Eur J Nutr 59:2011–2024. https://doi.org/10.1007/s00394-019-02050-0
Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, Piccini F (2019) Ketogenic diet and microbiota: friends or enemies? Genes 10:534. https://doi.org/10.3390/genes10070534
Parker HW, Vadiveloo MK (2019) Diet quality of vegetarian diets compared with nonvegetarian diets: a systematic review. Nutr Rev 77:144–160. https://doi.org/10.1093/nutrit/nuy067
Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A et al (2019) A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr Neurosci 22:474–487. https://doi.org/10.1080/1028415X.2017.1411320
Pawlak R (2017) Vegetarian diets in the prevention and management of diabetes and its complications. Diabetes Spectr 30:82–88. https://doi.org/10.2337/ds16-0057
Pedditzi E, Peters R, Beckett N (2016) The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing 45:14–21. https://doi.org/10.1093/ageing/afv151
Piernas C, Gao M, Jebb S (2022) Dietary patterns derived by reduced rank regression and non-communicable disease risk. Proc Nutr Soc 1–8. https://doi.org/10.1017/S0029665122001094
Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK et al (2010) Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 219:25–32. https://doi.org/10.1016/j.jneuroim.2009.11.010
Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N (2013) Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol 74:580–591. https://doi.org/10.1002/ana.23944
Rew L, Harris MD, Goldie J (2022) The ketogenic diet: its impact on human gut microbiota and potential consequent health outcomes: a systematic literature review. Gastroenterol Hepatol Bed Bench 15:326–342. https://doi.org/10.22037/ghfbb.v15i4.2600
Richardson LA, Izuora K, Basu A (2022) Mediterranean diet and its association with cardiovascular disease risk factors: a scoping review. Int J Environ Res Public Health 19:12762. https://doi.org/10.3390/ijerph191912762
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A et al (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7:14. https://doi.org/10.3390/microorganisms7010014
Sánchez-Villegas A, Martínez-González MA, Estruch R, Salas-Salvadó J, Corella D, Covas MI et al (2013) Mediterranean dietary pattern and depression: the PREDIMED randomized trial. BMC Med 11:208. https://doi.org/10.1186/1741-7015-11-208
Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF (2017) Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 179:223–244. https://doi.org/10.1016/j.trsl.2016.10.002
Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP et al (2015) Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2:271–274. https://doi.org/10.1016/S2215-0366(14)00051-0
Scalbert A, Brennan L, Manach C, Andres-Lacueva C, Dragsted LO, Draper J et al (2014) The food metabolome: a window over dietary exposure. Am J Clin Nutr 99:1286–1308. https://doi.org/10.3945/ajcn.113.076133
Schmidt JA, Rinaldi S, Scalbert A, Ferrari P, Achaintre D, Gunter MJ et al (2016) Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr 70:306–312. https://doi.org/10.1038/ejcn.2015.144
Seong J, Kang JY, Sun JS, Kim KW (2019) Hypothalamic inflammation and obesity: a mechanistic review. Arch Pharm Res 42:383–392. https://doi.org/10.1007/s12272-019-01138-9
Shegelman A, Carson KA, McDonald TJW, Henry-Barron BJ, Diaz-Arias LA, Cervenka MC (2021) The psychiatric effects of ketogenic diet therapy on adults with chronic epilepsy. Epilepsy Behav 117:107807. https://doi.org/10.1016/j.yebeh.2021.107807
Silva YP, Bernardi A, Frozza RL (2020) The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol 11:25. https://doi.org/10.3389/fendo.2020.00025
Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC et al (2014) Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 39:271–282. https://doi.org/10.3233/JAD-130830
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K et al (2017) Influence of diet on the gut microbiome and implications for human health. J Transl Med 15:73. https://doi.org/10.1186/s12967-017-1175-y
Stevenson RJ, Francis HM, Attuquayefio T, Gupta D, Yeomans MR, Oaten MJ et al (2020) Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R Soc Open Sci 7:191338. https://doi.org/10.1098/rsos.191338
Stokes N, Gordon CM, DiVasta A (2011) Vegetarian diets and mental health in adolescents with anorexia nervosa. J Adolesc Health 48:S50. https://doi.org/10.1016/j.jadohealth.2010.11.109
Storz MA, Ronco AL (2023) Adherence to a vegetarian diet is not associated with depression: results from the National Health and Nutrition Examination surveys. Psychiatry Investig 20:315–324. https://doi.org/10.30773/pi.2022.0251
Sudo N (2019) Biogenic amines: signals between commensal microbiota and gut physiology. Front Endocrinol 10:504. https://doi.org/10.3389/fendo.2019.00504
Tardy AL, Pouteau E, Marquez D, Yilmaz C, Scholey A (2020) Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients 12:228. https://doi.org/10.3390/nu12010228
Taylor AM, Thompson SV, Edwards CG, Musaad SMA, Khan NA, Holscher HD (2019) Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr Neurosci 22:1–10. https://doi.org/10.1080/1028415X.2019.1582578
Teasdale S, Mörkl S, Müller-Stierlin AS (2020) Nutritional psychiatry in the treatment of psychotic disorders: current hypotheses and research challenges. Brain Behav Immun Health 5:100070. https://doi.org/10.1016/j.bbih.2020.100070
Thanarajah SE, Backes H, Di Feliceantonio AG, Albus K, Cremer AL, Hanssen R et al (2019) Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans. Cell Metab 29:695-706.e4. https://doi.org/10.1016/j.cmet.2018.12.006
Tillery EE, Ellis KD, Threatt TB, Reyes HA, Plummer CS, Barney LR (2021) The use of the ketogenic diet in the treatment of psychiatric disorders. Ment Health Clin 11:211–219. https://doi.org/10.9740/mhc.2021.05.211
Timko CA, Hormes JM, Chubski J (2012) Will the real vegetarian please stand up? An investigation of dietary restraint and eating disorder symptoms in vegetarians versus non-vegetarians. Appetite 58:982–990. https://doi.org/10.1016/j.appet.2012.02.005
Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND et al (2019) The effects of vegetarian and vegan diets on gut microbiota. Front Nutr 6:47. https://doi.org/10.3389/fnut.2019.00047
Tosti V, Bertozzi B, Fontana L (2018) Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci 73:318–326. https://doi.org/10.1093/gerona/glx227
Trichopoulou A, Kyrozis A, Rossi M, Katsoulis M, Trichopoulos D, La Vecchia C et al (2015) Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr 54:1311–1321. https://doi.org/10.1007/s00394-014-0811-z
Valdes AM, Walter J, Segal E, Spector TD (2018) Role of the gut microbiota in nutrition and health. BMJ 361:36–44. https://doi.org/10.1136/bmj.k2179
Vallès Y, Artacho A, Pascual-García A, Ferrús ML, Gosalbes MJ, Abellán JJ et al (2014) Microbial succession in the gut: directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLoS Genet 10:e1004406. https://doi.org/10.1371/journal.pgen.1004406
Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MÁ et al (2015) Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med 175:1094–1103. https://doi.org/10.1001/jamainternmed.2015.1668
van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O (2019) The Mediterranean, dietary approaches to stop hypertension (DASH), and Mediterranean-DASH intervention for neurodegenerative delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease - a review. Adv Nutr 10:1040–1065. https://doi.org/10.1093/advances/nmz054
Varlamov O (2017) Western-style diet, sex steroids and metabolism. Biochim Biophys Acta Mol Basis Dis 1863:1147–1155. https://doi.org/10.1016/j.bbadis.2016.05.025
Wang X, Qi Y, Zheng H (2022) Dietary polyphenol, gut microbiota, and health benefits. Antioxidants 11:1212. https://doi.org/10.3390/antiox11061212
Wani AL, Bhat SA, Ara A (2015) Omega-3 fatty acids and the treatment of depression: a review of scientific evidence. Integr Med Res 4:132–141. https://doi.org/10.1016/j.imr.2015.07.003
Westman EC, Mavropoulos J, Yancy WS, Volek JS (2003) A review of low-carbohydrate ketogenic diets. Curr Atheroscler Rep 5:476–483. https://doi.org/10.1007/s11883-003-0038-6
Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ (2006) Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243. https://doi.org/10.1097/00004836-200603000-00015
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. https://doi.org/10.1126/science.1208344
Wu L, Sun D (2017) Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep 7:41317. https://doi.org/10.1038/srep41317
Xiao W, Zhang Q, Yu L, Tian F, Chen W, Zhai Q (2022) Effects of vegetarian diet-associated nutrients on gut microbiota and intestinal physiology. Food Sci Hum Wellness 11:208–217. https://doi.org/10.1016/j.fshw.2021.11.002
Xie G, Zhou Q, Qiu CZ, Dai WK, Wang HP, Li YH et al (2017) Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol 23:6164–6171. https://doi.org/10.3748/wjg.v23.i33.6164
Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L (2011) Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology 76:1568–1574. https://doi.org/10.1212/WNL.0b013e3182190d09
Yao Y, Cai X, Ye Y, Wang F, Chen F, Zheng C (2021) The role of microbiota in infant health: from early life to adulthood. Front Immunol 12:708472. https://doi.org/10.3389/fimmu.2021.708472
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M et al (2012) Human gut microbiome viewed across age and geography. Nature 486:222–227. https://doi.org/10.1038/nature11053
Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I (2008) Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia 49:73–75. https://doi.org/10.1111/j.1528-1167.2008.01841.x
Zamroziewicz MK, Barbey AK (2016) Nutritional cognitive neuroscience: innovations for healthy brain aging. Front Neurosci 10:240. https://doi.org/10.3389/fnins.2016.00240
Zanchi D, Depoorter A, Egloff L, Haller S, Mählmann L, Lang UE et al (2017) The impact of gut hormones on the neural circuit of appetite and satiety: a systematic review. Neurosci Biobehav Rev 80:457–475. https://doi.org/10.1016/j.neubiorev.2017.06.013
Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A et al (2015) Personalized nutrition by prediction of glycemic responses. Cell 163:1079–1094. https://doi.org/10.1016/j.cell.2015.11.001
Zhang C, Björkman A, Cai K, Liu G, Wang C, Li Y et al (2018a) Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol 9:908. https://doi.org/10.3389/fimmu.2018.00908
Zhang Y, Zhou S, Zhou Y, Yu L, Zhang L, Wang Y (2018b) Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res 145:163–168. https://doi.org/10.1016/j.eplepsyres.2018.06.015
Zinöcker MK, Lindseth IA (2018) The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients 10:365. https://doi.org/10.3390/nu10030365
Funding
Funding for open access publishing: Universidad Málaga/CBUA
Author information
Authors and Affiliations
Contributions
Alejandro Borrego-Ruiz: conceptualization, methodology, writing (review and editing). Juan J. Borrego: investigation, resources, writing (original draft).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borrego-Ruiz, A., Borrego, J.J. Human gut microbiome, diet, and mental disorders. Int Microbiol (2024). https://doi.org/10.1007/s10123-024-00518-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10123-024-00518-6