Abstract
Bacteriophages have been mainly used in treating infections caused by planktonic bacterial cells in the veterinary sector. However, their applications as antibiofilm agents have received little attention. Accordingly, a previously isolated Salmonella infecting Siphoviridae phage was investigated for host range against 15 Salmonella enterica isolates (S. Cape, S. Gallinarum, 4 S. Enteritidis, 3 S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, S. Agona, S. Daula, and S. Aba) recovered from the litters of commercial broiler farms. All S. enterica isolates were examined for their biofilm activity using a microtiter plate assay and for adrA, csgD, and gcpA genes using conventional PCR. The phage efficacy against established biofilms produced by the selected seven S. enterica isolates (S. Gallinarum, S. Enteritidis, S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, and S. Agona) was assessed using microtiter plate assay and reverse transcriptase real-time PCR over different incubation times of 5 and 24 h. All S. enterica isolates were strong biofilm formers. Moreover, the phage effectively reduced the biofilm activity of the established S. enterica biofilms in the microtiter plate assay using the independent sample t-test (P < 0.050). Furthermore, the relative expression levels of csgD, gcpA, and adrA genes in the biofilm cells of S. enterica isolate after phage treatment were significantly up-regulated to variable degrees using the independent sample t-test (P < 0.050). In conclusion, the present study revealed the potential use of Salmonella phage in reducing established biofilms produced by S. enterica serovars isolated from broiler farms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonellosis is one of the most common diseases affecting poultry that has a significant economic impact on the poultry industry and poses a major threat to public health worldwide (Cosby et al. 2015). The genus Salmonella consists of more than 2500 serotypes. The diversity of Salmonella serotypes reported from poultry sources is based on geographical distribution and time change. However, several serotypes, such as S. Enteritidis, S. Typhimurium, S. Infantis, S. Newport, and S. Derby, have been reported at a high incidence (Khan et al. 2018; Merino et al. 2019).
Bacterial biofilms are complex microbial communities established on various biotic and abiotic surfaces that are generally enclosed in an extracellular matrix composed of different biopolymers (Hosseinidoust et al., 2013). Biofilm formation can be considered one of the cellular survival mechanisms that make cells more resistant to adverse environmental conditions and provide resistance against different antibiotic intervention regimens (Kirketerp-Møller et al. 2008; Hosseinidoust et al. 2013). Approximately 50% of the Salmonella strains isolated from poultry farms produced biofilms in the processing areas of poultry farms and contact surfaces (Wang et al. 2013; Merino et al. 2019). The adhesion of S. enterica on surfaces is mainly controlled by the presence of biofilm-specific genes such as csgD, adrA, and gcpA related to the synthesis of cellulose and curli fimbriae (García et al. 2004; Bhowmick et al. 2011). The co-expression of fimbriae and cellulose contributes to forming a tightly packed cell matrix enclosed in a hydrophobic network, which is crucial for biofilm formation and tolerance to disinfectants on different surfaces (Jain and Chen 2007). As bacterial biofilms show high antibiotic resistance, eradicating established biofilms is based on using compounds that can penetrate or disrupt them mechanically (Paluch et al. 2020). The most common strategy used for combating microbial adhesion and biofilm formation in poultry houses is based on the chemical attack through cleaning and disinfection procedures. However, these procedures are not fully effective in biofilm eradication (Garcia et al. 2017). The phage-based approach has been studied as an alternative biological tool for biofilm prevention and control (Carson et al. 2010; Kelly et al. 2012). Certain phages can dissolve the extracellular matrix of biofilms by either producing exopolysaccharide-degrading enzymes (EPS-degrading enzymes) such as depolymerases, lysins, DNases, quorum-quenching enzymes, and lipases or inducing the production of EPS-degrading enzymes by bacterial hosts under stress triggered by phage infection ( Maciejewska et al. 2018; Chegini et al. 2020).
Previous investigations have reported the effectiveness of bacteriophages in the inhibition and eradication of the established biofilms produced by clinical Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, and Klebsiella pneumoniae (Bedi et al. 2009; Carson et al. 2010; Ahiwale et al. 2011; Kelly et al. 2012).
However, their veterinary applications as antibiofilm agents still have minimal studies. Earlier investigations were mainly concerned with their applications in treating and preventing different infections caused by planktonic cells (Fiorentin et al. 2005; Lau et al. 2010; Nabil et al. 2018).
The results of the only available study by Garcia et al. (2017) have displayed the effectiveness of bacteriophage in the inhibition of biofilm formation in biofilm-forming S. enterica serovars (S. Enteritidis, S. Heidelberg, S. Kentucky, S. Senftenberg, and S. Typhimurium) on surfaces present in chicken slaughterhouses (Garcia et al. 2017). The research on the phage effect on eradicating established S. enterica biofilms, including the underlying molecular expression patterns, was given little attention. Therefore, this study aims to provide an evaluation report on the antibiofilm activity of a previously isolated Salmonella phage against different strong biofilm former S. enterica serovars (S. Gallinarum, S. Enteritidis, S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, and S. Agona) isolated from litters of commercial broiler farms using microtiter plate assay and differential expression of the biofilm-associated genes.
Materials and methods
Isolation and identification of S. enterica serovars
Between November 2019 and February 2020, 210 pooled litter samples were collected from 42 commercial broiler farms (five samples/farm) in the Giza governorate. The samples were examined for the isolation of S. enterica serovars in the Reference Laboratory for Veterinary Quality Control on Poultry Production, Dokki, Egypt (RLQP, Egypt). The isolation was performed according to ISO6579-1 (2017) by inoculating 10 g of samples into 90 ml of iso buffer peptone water (Oxoid Limited, Thermo Fisher Scientific Inc., UK), and incubating at 37°C for 24 h. After that, 0.1 ml of the enriched sample was inoculated into 9.9 ml of modified semisolid Rappaport-Vassiliadis medium (RV) (LabM, Bury, Lancashire, UK), and incubated at 41.5°C for 24 h. Furthermore, 1 ml of the enriched sample was transferred to Muller-Kauffmann tetrathionate-novobiocin broth (MKTT) (LabM, Bury, Lancashire, UK), and incubated at 37°C for 24 h. A loopful of two tubes was then streaked onto xylose lysine deoxycholate agar (XLD) (LabM, Bury, Lancashire, UK), and brilliant green agar (BG) (Oxoid Limited, Thermo Fisher Scientific Inc., UK) and incubated at 37°C for 24 h. The plates were examined for the presence of red colonies with black centers on XLD agar and red colonies surrounded by a red halo on BG agar. The suspected Salmonella colonies were identified according to (ISO6579-1 2017) using different biochemical tests such as triple sugar iron agar, urea, indole, and lysine iron (Oxoid Limited, Thermo Fisher Scientific Inc., UK). Serotyping of Salmonella isolates was conducted according to ISO6579-3 (2014) through the identification of somatic (O) and flagellar (H) antigens using SIFIN antisera (Berlin, Germany) located at the RLQP, Egypt.
Bacteriophage propagation
The phage used in this study was a lytic Siphoviridae Salmonella phage previously isolated from poultry litter at a commercial broiler farm in the Giza governorate in 2019 (Sorour et al. 2020).
The phage host propagating strain used in this study was S. Kentucky, which was previously isolated from a commercial broiler farm in Giza governorate with severe gross lesions such as enteritis, trachitis, pneumonia, airsacculitis, perihepatitis, pericarditis, nephritis, and typhlitis (Sorour et al. 2020). The phage lysate was amplified, as described by Nabil et al. (2018) and Sorour et al. (2020). Briefly, 4.5 ml of phage lysate (108 PFUml−1) was mixed with 0.5 ml mid-exponential S. Kentucky culture (OD600 = 0.45) and 0.5 ml buffer peptone broth (Oxoid Limited, Thermo Fisher Scientific Inc., UK) in a falcon tube (Techno Plastic Products, Transadingen, Switzerland) and incubated at 37°C for 24 h. The mixture was centrifuged at 11,200 ×g for 10 min, and the supernatant was filtered through a 0.45 μm pore syringe filter (Corning, NY, Germany). After that, the phage lysate (108 PFUml−1) was tested using tryptic soy agar plates (Oxoid Limited, Thermo Fisher Scientific Inc.) by the spot test and plaque assay as described by Sorour et al. (2020). Phage lysate was then purified three times, as described by Hosny et al. (2021), using the plaque assay method with modification in the broth medium. A single clear plaque was picked into 300 μl of buffer peptone broth, followed by centrifugation and filtration as previously described in the amplification method. The purified phage lysate was stored at 4°C.
Bacteriophage host range using spot test assay
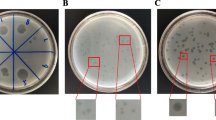
According to Rahaman et al. (2014), the host range of Salmonella phage was tested in vitro against 15 recovered S. enterica isolates. Ten microliters of the phage lysate (108 PFUml−1) were spotted on tryptic soy agar plates (Oxoid Limited, Thermo Fisher Scientific Inc., UK) overlaid with 15 S. enterica isolates. After that, the plates were incubated at 37°C for 24 h and examined for the presence of lytic zones of bacterial growth.
Bacteriophage efficiency of plating (EOP)
The efficiency of the plating test was determined against 15 recovered S. enterica isolates using the plaque assay as described by Vongkamjan et al. (2017) to confirm host-range profiles of Salmonella phages obtained from the spot test assay. One hundred microliters of ten folded dilutions of phage lysate (101 to 1010) were mixed with 100 µl of the host S. Kentucky and the tested isolates. After that, 3 ml of molten soft agar (13 g/l of nutrient broth and 28 g/l of nutrient agar) (HiMedia, Pvt. Ltd., India) were added to the mixture, and overlaid onto tryptic soya agar plates. The titers were determined after 24 h of incubation. Single plaques were counted and expressed as plaque-forming units (PFUml−1). EOP was calculated according to the equation: (EOP = average phage titer on the tested isolate/average phage titer on the host isolate). The obtained EOP values were classified as described by Manohar et al. (2019) as high (EOP ≥ 0.5), medium (0.5 < EOP ≥ 0.1), low (0.1 < EOP ˃ 0.001), and inefficient (EOP ≤ 0.001).
Screening for adrA, gcpA, and csgD biofilm-associated genes in S. enterica isolates using a conventional polymerase chain reaction
The DNAs of 15 S. enterica isolates were extracted using a QIAGEN kit (Qiagen, Germany, GmbH). Table S1 summarizes the primer sequences and amplicon sizes used to detect adrA, gcpA, and csgD genes. The specificity of primers was tested using a negative control of C. perfringens ATCC 12917 and positive control of a locally isolated S. Enteritidis strain containing adrA, gcpA, and csgD genes obtained from the ISO 17025 accredited biotechnology unit, RLQP, Egypt (Nabil and Yonis 2019). Each PCR reaction was performed in a 50 µl total reaction volume containing 25 µl of Emerald Amp® Max PCR master mix (Takara Bio Inc, Japan), 1 µl (20 pmol) of each primer, 11 µl of DNAse and RNAse free water, and 6 µl of genomic DNA extract. The amplification was conducted according to Bhowmick et al. (2011) in an Applied Biosystems 2720 Thermal Cycler (Applied Biosystems). The PCR conditions were 94°C for 5 min for initial denaturation, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 50°C for adrA and csgD genes, and 57°C for gcpA for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 5 min. The amplified fragments were analyzed in a 1.2% agarose gel stained with 0.2 μg/ml ethidium bromide.
Detection of the biofilm-forming ability of the S. enterica isolates
A total of 15 S. enterica isolates (S. cape, S. Gallinarum, 4 S. Enteritidis, 3 S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, S. Agona, S. Daula, and S. Aba) were examined for their ability for biofilm formation using the microtiter plate assay method. Biofilm formation was quantified using a 96-well flat-bottomed polystyrene microtiter plate (Greiner Bio-One, Germany) as described by Stepanović et al. (2004). Briefly, exponential growth-phase bacteria were prepared by inoculating 107 CFUml−1 of each S. enterica isolate in Luria-Bertani (LB) broth (Sigma-Aldrich, St. Louis, USA) and incubated at 37°C for 4 h (Spoering and Lewis 2001; Zadeh et al. 2022). After that, 200 μl of bacterial suspensions were added to each well, and the plate was incubated at 37°C for 24 h. Negative control wells contained 200 μl of LB broth alone without bacterial culture. The wells were then poured off and washed three times with sterile phosphate-buffered saline (PBS) (Sigma-Aldrich, St. Louis, USA). The attached bacteria were fixed with 200 μl of 99% methanol (Merck, Darmstadt, Germany) for 15 min, and the wells were then emptied and air-dried. The wells were stained with 200 μl of 0.1% crystal violet solution (Sigma-Aldrich, St. Louis, USA) for 5 min. The stain was rinsed off, and the wells were washed under running tap water. The wells were then left for 15 min for air drying and resolubilized with 200 μl of 33% (v/v) glacial acetic acid. This assay was conducted three times for all S. enterica isolates and negative control. Each well’s optical density (OD) was measured at 620 nm with an ELISA reader serial no: 610000079 (Tecan Sunrise, Jencons, UK). S. enterica isolates were classified into four degrees of biofilm formation based on the optical absorbance of negative control (ODc): non-biofilm producer (OD ≤ ODc), weak biofilm producer (ODc < OD ≤ 2×ODc), moderate biofilm producer (2×ODc < OD ≤ 4×ODc), and strong biofilm producer (4×ODc < OD). All measurements were expressed as the mean ± standard deviations.
Detection of antibiofilm activity of Salmonella phage against established S. enterica biofilms
The evaluation of the phage efficiency in the biofilm control was assessed against established biofilms produced by seven strong biofilm producers of S. enterica isolates (S. Gallinarum, S. Enteritidis, S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, and S. Agona) that had OD values of 0.61, 0.78, 0.67, 0.62, 0.61, 0.62, and 0.59, respectively, as described by Chibeu et al. (2012). The filtered phage lysate was diluted in LB broth to obtain five concentrations (101, 103, 105, 107, and 109 PFUml−1). Briefly, 200 μl of each diluted phage lysate was added to the established biofilms produced on the microtiter plates by seven S. enterica isolates (ten microtiter plates; five plates used for microtiter assay and other five used for gene expression). Negative control wells contained established bacterial biofilms inoculated with 200 μl of LB broth without the addition of phage. After that, the plates were incubated at 37°C for 5 and 24 h to assess the time-dose effect of phage application on the established biofilms. The contents of the wells were transferred into tryptic soy agar plates for phage titration after treatments, and the wells were then washed three times with sterile phosphate-buffered saline (PBS) (Sigma-Aldrich, St. Louis, USA). The remaining biofilm cells were stained as previously described to determine the final biofilm density. The optical density of each well was measured as previously described at 620 nm with an ELISA reader serial no: 610000079 (Tecan Sunrise, Jencons, UK). This assay was conducted three times against all tested S. enterica isolates and negative controls. All measurements were expressed as the mean ± standard deviations.
RNA extraction from the established S. enterica biofilms after phage treatment
The phage-treated S. enterica biofilms and negative control samples (established bacterial biofilms and LB media without the addition of phage) were scraped three times using a sterile cell scraper (Greiner Bio-One, Germany) from the microtiter plates after 5 and 24 h of treatment and collected in a cold, sterile double-distilled water falcon tube. Samples were then centrifuged for 5 min at 3345 ×g at 4°C. Pellets were collected and resuspended in RLT buffer (Qiagen GmbH, Hilden, Germany) and disrupted at high speed (30 Hz) with the Qiagen Tissue Lyser for 2 min. Total RNAs of the collected samples were extracted using the RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. They were subsequently treated with RNase-Free DNase (Qiagen GmbH, Hilden, Germany) to remove the remaining DNA.
Quantification of the expression levels of adrA, gcpA, and csgD genes in the established S. enterica biofilms after phage treatment
Reverse transcriptase real-time PCR was used to quantify the level of expression of csgD, adrA, and gcpA genes. The real-time PCR data in this study were represented relative to the 16S rRNA gene as a housekeeping gene (Yang et al. 2014). Primer sequences for amplifying the genes adrA, gcpA, and csgD were outlined in Table S1. Real-time PCR was performed in the Stratagene MX3005P Real-Time System thermal cycler according to Bhowmick et al. (2011) in a total volume of 25 µl, consisting of 12.5 µl of 2x QuantiTect SYBR green master mix (Qiagen, Germany, GmbH), 0.25 µl of RevertAid Reverse Transcriptase (200 U/µl) (Thermo Fisher), 0.5 µl of each primer (20 pmol), 8.25 µl of water, and 3 µl of RNA template. All reactions were performed in triplicates. The thermocycler conditions used for the amplification included an initial reverse transcription at 50°C for 30 min, initial denaturation at 94°C for 15 min followed by 40 cycles of denaturation at 94°C for 15 s, annealing at 60°C for 16s rRNA and 50°C for adrA and csgD genes, and 57°C for gcpA for 30 s, and extension at 72°C for 1 min. A dissociation curve was generated for each gene by denaturation at 94°C for 1 min, annealing at 60°C for 16s rRNA and 50°C for adrA and csgD genes, and 57°C for gcpA for 1 min, and final denaturation at 94°C for 1 min. Data acquisition of the amplification curves and CT values were determined by the Stratagene MX3005P software. Gene expression was calculated as described by Livak and Schmittgen (2001) using the ΔΔCt formula. The expression of the genes was normalized to the 16s rRNA gene as a housekeeping gene by calculating ΔCT. The ΔCT of each sample was compared with that of the negative control group by calculating ΔΔCT. The results of target gene expression were displayed as n-fold changes in the transcription level relative to the negative control group.
Statistical analysis
The data were analyzed using IBM SPSS Statistics (Version 21.0, Armonk, NY, IBM Corp.). All optical density and quantitative real-time PCR data were log10-transformed before statistical analyses. An independent sample t-test was used to determine the difference in the biofilm eradication and expression levels of biofilm-associated genes between the negative control and the phage-treated groups. The effect of serotyping, phage concentration and their interaction on biofilm eradication was evaluated using a two-way ANOVA with LSD post hoc test. A paired sample t-test was used to determine the difference in the biofilm eradication and expression levels of biofilm-associated genes after phage treatment over incubation periods of 5 and 24 h. P-values were considered significant at a level ≤ 0.050.
Results
Isolation and identification of S. enterica serovars
Among the examined 210 pooled litter samples, the prevalence of salmonellosis was 35.7% (75/210), representing 15 isolates. The biochemical analysis of the suspected Salmonella colonies revealed that they were resolved to be Salmonella species when they were positive for lysine iron and H2S production in triple sugar iron. Meanwhile, they were negative for urea and indole. The identified isolates were S. Cape, S. Gallinarum, 4 S. Enteritidis, 3 S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, S. Agona, S. Daula, and S. Aba (Table 1).
Bacteriophage host range using spot test assay and efficiency of plating
The Salmonella phage showed a broad host range with lytic ability against all S. enterica isolates (100%) using a spot test assay. Salmonella phage had a high efficiency (˃1) against all S. enterica isolates (100%). The EOP of Salmonella phage in S. Cape, S. Gallinarum, S. Montevideo, S. Uno was 1.1, while in S. Enteritidis, it ranged from 1.06 to 1.08. The EOP of Salmonella phage in S. Oritamerin, S. Belgdam, S. Agona, S. Daula, S. Aba was 1.2, 1.07, 1.09, 1.03, and 1.01, respectively (Table 1).
Screening for adrA, gcpA, and csgD biofilm-associated genes in S. enterica isolates using a conventional polymerase chain reaction
PCR analysis yielded the detection of adrA (1113-bp), csgD (651-bp), and gcpA (1713-bp) genes in all S. enterica isolates (Fig. S1a, b, and c).
Detection of the biofilm-forming ability of the S. enterica isolates using a microtiter plate assay
All S. enterica isolates tested in this study produced strong biofilms on a polystyrene microtiter plate. The absorbance ranged from 0.58 to 0.78, and a cutoff value (ODc) of 0.12 was used for isolate classification. Isolates were considered strong biofilm formers as the measured OD620 was greater than 4-fold the value obtained in the negative control (Table S2).
Detection of antibiofilm activity of Salmonella phage against established S. enterica biofilms in the microtiter plate assay
Overall, the Salmonella phage was efficient in a significant reduction in the biofilm mass of established S. enterica biofilms over incubation periods of 5 and 24 h in the microtiter plate assay when compared to the negative control group using an independent sample t-test (P = 0.002 and 0.000, respectively) (Fig. 1a b and Tables S3 and S4). Furthermore, the titers of Salmonella phage had increased after 5 and 24 h of treatments at all phage dilutions (101, 103, 105, 107, and 109). This indicates that phage infection and replication were successful (Table S5). A two-way ANOVA was employed to examine the effect of serotyping and phage doses over 5 and 24 h of incubation on biofilm eradication. There was a statistically significant interaction between the effects of serotyping and phage doses on biofilm reduction (p = 0.000). Simple main effect analysis using the LSD post hoc test revealed that there were no differences in the biofilm reduction between S. Montevideo and S. Belgdom (P = 0.137), S. Montevideo and S. Agona (P = 0.067), S. Oritamerin and S. Uno (P = 0.616) compared to the remaining S. enterica isolates that showed significant differences over an incubation period of 5 h (P < 0.050). Furthermore, there were significant differences in the biofilm reduction in all S. enterica isolates over an incubation period of 24 h (P < 0.050). There were significant differences in the biofilm reduction at all phage concentrations over incubation periods of 5 and 24 h (P < 0.050).
Effect of Salmonella phage on the established S. enterica biofilms after 5 h (a) and 24 h (b) of treatment. Phage was effective in the reduction of established biofilms produced by (S. Gallinarum, S. Enteritidis, S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, and S. Agona) compared to untreated negative controls in a time-dose-dependent manner. The data are expressed as mean of three replicated measurements ± standard deviation (represented by error bars). Different letters (a, b, c, d, e, and f) indicate significant differences in the biofilm eradication among isolates based on two-way ANOVA with LSD post hoc test. Isolates that have the same letters are significantly different in the biofilm eradication from each other. P-values were considered significant at a level ≤ 0.050
The highest mean difference for the biofilm reduction was observed at phage concentration (109 PFUml−1) relative to negative control over incubation periods of 5 and 24 h as 0.242 and 0.503, respectively, compared to other phage concentrations (101, 103, 105, and 107 PFUml−1). These findings suggest that Salmonella phage effectively reduced the established biofilms produced by seven S. enterica isolates in a dose-dependent manner over incubation periods of 5 and 24 h. A paired sample t-test revealed a significant difference in the biofilm reduction after phage treatment over incubation periods of 5 and 24 h (P = 0.000). The biofilm reduction after phage treatment over an incubation period of 24 h was 0.245, higher than the incubation period of 5 h.
Quantification of the expression level of adrA, gcpA, and csgD genes in the established S. enterica biofilms after phage treatment using reverse transcriptase real-time PCR
The overall analysis of the biofilm-associated genes (csgD, gcpA, and adrA) in the established S. enterica biofilms revealed significant increments at variable degrees in a time-dose-dependent manner after phage treatment over incubation periods of 5 and 24 h compared to the negative control group using an independent sample t-test (P < 0.050). A paired sample t-test revealed a significant difference in the expression levels of biofilm-associated genes (csgD, gcpA, and adrA) after phage treatment over incubation periods of 5 and 24 h (P ≤ 0.050) (Fig. 2a, b).
a, b Relative gene expression of csgD, gcpA and adrA genes in the established S. enterica biofilms after 5 and 24 h of phage treatment. A paired sample t-test revealed a significant difference in the expression levels of csgD, gcpA and adrA genes after 5 and 24 h of phage treatment (P ≤ 0.050). The data are expressed as mean of three replicated measurements for each gene at all phage concentrations ± standard deviation (represented by error bars)
The expression of csgD and adrA genes in established biofilms produced by six S. enterica isolates (S. Gallinarum, S. Enteritidis, S. Montevideo, S. Oritamerin, S. Belgdom, S. Agona) following exposure to phage treatment for 5 h revealed elevated levels of expression at concentrations of 101, 103, 105, 107, and 109 PFUml−1. Meanwhile, their expression in the established biofilm produced by S. Uno increased at concentrations of 101, 103, 105, and 107 PFUml−1 with no further increment at 109 PFUml−1. Prolonged treatment for 24 h revealed an increase in the expression levels of csgD and adrA genes in S. Enteritidis, S. Montevideo, S. Belgdom, S. Uno, and S. Agona at concentrations of 101, 103, 105, 107, and 109 PFUml−1 compared to the remaining serotypes, S. Gallinarum, and S. Oritamerin that showed an increase in the expression at concentrations of 101, 103, 105, and 107 PFUml−1 with no further increment at 109 PFUml−1 (Fig. 3a, b).
Mean fold change of the expression level of (a) csgD, (b) adrA, (c) gcpA genes in the biofilm forming S. enterica isolates treated with different concentrations of phage for 5 and 24 h. Phage was upregulated the expression levels of csgD, adrA, and gcpA genes in the established S. enterica biofilms at variable degrees in a time-dose-dependent manner. Fold change was calculated as mean of three replicated measurements ± standard deviation (represented by error bars)
The relative expression level of the gcpA gene in all established biofilms produced by seven S. enterica isolates after exposure to phage treatment for 5 h revealed an elevated expression level at concentrations of 101, 103, 105, 107, and 109 PFUml−1. Prolonged treatment for 24 h revealed an increase in the expression of the gcpA gene in the established biofilms produced by S. Enteritidis and S. Belgdom at concentrations of 101, 103, 105, 107, and 109 PFUml−1 compared to the remaining serotypes, S. Gallinarum, S. Montevideo, S. Uno, S. Agona, and S. Oritamerin that showed an increase in the expression at concentrations of 101, 103, 105, and 107 PFUml−1, with no further increment at 109 PFUml−1 (Fig. 3c).
Discussion
Bacteriophages are viruses infecting bacteria identified as antibiotic alternatives (Dalmasso et al. 2016). Earlier clinical studies have displayed the potential use of bacteriophages in biofilm control and eradication; however, their applications as antibiofilm agents in the veterinary field have not been reported (Bedi et al. 2009; Carson et al. 2010; Ahiwale et al. 2011; Kelly et al. 2012).
The Salmonella phage used in this study has previously been isolated, characterized, and assessed for its potential role in the prevention and treatment of S. Kentucky infection in broilers (Sorour et al., 2020). However, this Salmonella phage has never been evaluated for the treatment of established biofilms produced by different S. enterica serovars, and thus, this is worthy of our investigation.
In this study, ten different Salmonella serovars were identified (S. Cape, S. Gallinarum, S. Enteritidis, S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, S. Agona, S. Daula, and S. Aba). The most prevalent Salmonella serovars were S. Enteritidis (26.7%) and S. Montevideo (20%). Similarly, previous studies conducted by Cheong et al. (2007) and Kim et al. (2012) have displayed a high incidence of several S. enterica serotypes in broiler chickens, such as S. Enteritidis (21.9% and 57.4% ), S. Typhimurium (23.4% and 6.4%), S. Montevideo (9.4% and 31.9%), respectively (Cheong et al. 2007; Kim et al. 2012). Furthermore, earlier research has reported that some Salmonella serotypes, such as S. Enteritidis, S. Typhimurium, S. Infantis, S. Newport, and S. Derby are the most recovered ones associated with poultry-associated infections (Álvarez-Fernández et al. 2012; Khan et al. 2018). Our study provided evidence of the prevalence of Salmonella in the litter samples (35%). This finding is consistent with a previous study by Shang et al. (2018). They reported that environmental samples such as poultry litter, feed, air, and fans are important reservoirs for Salmonella infections in poultry farms.
The lytic profile of the Salmonella phage was determined against 15 S. enterica isolates using spot test and the efficiency of plating assay. The phage lysed all S. enterica isolates (S. Cape, S. Gallinarum, 4 S. Enteritidis, 3 S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, S. Agona, S. Daula, and S. Aba) with high efficiency of plating (˃1). The broad host range characteristic of Salmonella phage observed in this study suggests its suitability for biocontrol applications. According to Bielke et al. (2007), not all bacteriophages are host-specific and genera-restricted. This may be useful for selecting bacteriophages with broad host ranges for the pathogen of interest. Pelyuntha et al. (2021) reported that Salmonella phages WP109 and 110 showed high EOP on S. Kentucky, S. Saintpaul, S. Enteritidis, and S. Albany. Gomez-Garcia et al. (2021) showed that Salmonella phage S1 showed high EOP on S. Pullorum, S. Gallinarum, and S. Enteritidis. Esmael et al. (2021) demonstrated that Salmonella phage cocktails SPHG1 and SPHG3 showed high EOP against all S. Typhimurium tested. Mahmoud et al. (2018) reported the ability of Siphoviridae bacteriophages (Salmacey1 and Salmacey2) to lyse four different Salmonella serovars; S. Typhimurium, S. Enteritidis, S. Kentucky, and S. Typhi using a spot test assay. Furthermore, Bielke et al. (2007) demonstrated the ability of a wide host range phage (WHR 8) to lyse 6 different Salmonella serovars; S. Montevideo, S. Heidelberg, S. Ohio, S. Typhimurium, S. Agona, and S. Minnesota using a spot test assay.
All S. enterica isolates were examined for their biofilm-forming activity using a microtiter plate assay and conventional PCR. The microtiter plates used in the biofilm assay mimic plastic materials commonly used in poultry farms (Borges et al. 2018). Our results showed that all S. enterica isolates could produce strong biofilm on a polystyrene microtiter plate. These results agree with earlier studies that reported the adherence of S. enterica serotypes to hydrophobic surfaces such as polystyrene (Tondo et al. 2010; Borges et al. 2018). Agarwal et al. (2011) demonstrated that intrinsic characteristics in Salmonella serovars, such as fimbriae, flagella, membrane proteins, and other cellular appendages, play a role in their biofilm activity. Borges et al. (2018) displayed the ability of S. Agona and S. Montevideo serotypes to be good biofilm producers. Molecular characterization revealed positive amplification for (adrA, csgD, and gcpA) genes in all examined S. enterica isolates. The selection of these genes in this study was based on results of earlier studies that reported the presence of biofilm-specific genes, such as csgD, adrA, and gcpA that play a role in the adherence of S. enterica on surfaces through involvement in the production of cellulose and the expression of curli fimbriae (García et al. 2004; Bhowmick et al. 2011). Bhowmick et al. (2011) reported similar observations regarding detecting these genes in different Salmonella serovars, such as S. Weltevreden, S. Enteritidis, and S. Typhimurium.
The Salmonella phage action was assessed in different concentrations against established biofilms produced by seven strong biofilm formers S. enterica isolates (S. Gallinarum, S. Enteritidis, S. Montevideo, S. Uno, S. Oritamerin, S. Belgdam, and S. Agona) using a microtiter plate assay over incubation times of 5 and 24 h. A time-dose-dependent reduction in the established biofilms produced by seven S. enterica isolates was observed over incubation for 5 and 24 h of treatment. It could mean that phage’s high titer (109 PFUml−1) was the most effective in reducing established biofilms after 5 and 24 h of treatment. Similarly, a study by Cornelissen et al. (2011) has displayed a time-dose-dependent reduction in Pseudomonas putida biofilm mass with a maximum reduction of 8 h after the addition of the high φ15 phage doses (104 and 106 PFUml−1). A study by Mapes et al. (2016) has displayed a significant reduction of clinically established Pseudomonas aeruginosa biofilms in a dose-dependent manner after 6 h of exposure to phage cocktail treatment.
On the other hand, Chibeu et al. (2012) demonstrated that vB_EcoP_ACG-C91, vB_EcoM_ACG-C40, and vB_EcoS_ACG-M12 phages significantly reduced the established biofilms produced by uropathogenic Escherichia coli over treatment for 8 h in a dose-independent manner. A study by Cerca et al. (2007) has demonstrated that Staphylococcus bacteriophage K (108 PFUml−1) significantly reduced established S. epidermidis biofilms after 24 h of treatment. Until now, there have been no published reports on the antibiofilm activity of Salmonella phage against S. enterica serotypes recovered from poultry samples. This study observed that phage was not equally eradicating the established biofilms produced by S. enterica isolates based on LSD post hoc test results. This might be attributed to the variation in the serotypes. Phage treatment of biofilms for 24 h resulted in a reduction of the established biofilms produced by S. Enteritidis and S. Belgdam at concentrations of 101, 103, 105, 107, and 109 PFUml−1 compared to the remaining serotypes, S. Gallinarum, S. Montevideo, S. Uno, S. Agona, and S. Oritamerin that showed a reduction of the established biofilms at concentrations of 101, 103, 105, and 107 PFUml−1, with no further decrease at 109 PFUml−1. These findings may indicate the re-establishment of the biofilms at higher phage concentrations ˃ 109 PFUml−1 over an incubation time of 24 h. Previous studies have displayed an increase in the biofilm mass produced by Pseudomonas aeruginosa and uropathogenic E. coli after 24 h of phage treatment. This may be attributed to the development of phage-resistant variants (Pires et al. 2011; Chibeu et al. 2012). Chibeu et al. (2012) reported that combining phage treatment with chemical antimicrobials could be an effective technique for preventing phage-resistant cells from re-establishing biofilms.
The quantification of specific messenger RNA was evaluated by reverse transcriptase real-time PCR to assess specific changes in the expression patterns of the biofilm-associated genes (adrA, csgD, and gcpA) in the established S. enterica biofilms after phage treatment. The molecular findings revealed that the phage treatment for 5 and 24 h up-regulated the expression of adrA, csgD, and gcpA genes in a time-dose-dependent manner in the established biofilms produced by seven S. enterica isolates. It could mean that the highest expression level was reported at phage titer (109 PFUml−1). This might relate to the bacterial survival levels in biofilms that could be explained by the presence of persistent cells within the biofilm that increased by an increment of phage concentration. This possibility complies with the microtiter plate assay results that revealed the phage’s ability to reduce, not eradicate, the established S. enterica biofilms over 5 and 24 h. Biofilms represent a potential reservoir for persistent bacteria, a non-heritable phenotypic variation in the bacterial population that displays high antibiotic tolerance by entering a slow-growth or non-growth state (Harms et al. 2017; Drescher et al. 2019). A study by Drescher et al. (2019) reported that persistent cells could spontaneously arise in a microbial population or be triggered by stressors such as antimicrobials. The formation of persistent cells is mediated by different mechanisms that either act alone or overlapped, such as stress response, toxin-antitoxin, energy production, and phosphate metabolism (Drescher et al. 2019).
Furthermore, previous studies have displayed that the number of persistent cells in biofilm increased in the older biofilms compared to the younger ones (Abedon 2016; Ferriol-Gonzalez and Domingo-Calap 2020). Accordingly, the suggestion of the presence of persistent cells within the biofilm in this study may be attributed to the response mechanism of bacteria to the phage treatment and the old biofilm age. Harper et al. (2014) reported that bacteriophages could infect persistent cells but remain dormant until they revert to normal cells and initiate a productive lytic infection. Therefore, phages can target persistent cells within biofilm through antibiotic treatment (Chegini et al. 2020). Li et al. (2021) reported that combining phage with antibiotic treatment can significantly destroy the bacterial biofilms, release the persistent cells into the nutrient environment, and subsequently enhance the metabolic activity of persistent cells, making them more sensitive to the phage treatment.
Study limitations
The limitation of this study is that the antibiofilm activity of the phage was assessed against only seven S. enterica isolates; this was attributed to the scarce resources in our country. Moreover, the limitations of the molecular method in this study are the lack of assessment of the molecular mechanisms behind the persistent cell formation and other biofilm-associated genes such as csgA, BcsA, RpoS, CrL, OmpR, and MlrA that play a role in the production of cellulose and the expression of curli fimbriae in S. enterica species (Chen et al. 2021).
Conclusions
The present study has displayed the potential use of Salmonella phage in reducing established biofilms produced by S. enterica serovars isolated from broiler farms. Furthermore, this study is the first to identify transcriptional expression patterns of the biofilm-associated genes after phage treatment. Further research studies are needed to assess the efficacy of Salmonella phage in preventing biofilm formation for comparison with the results of this study that have assayed its efficacy in eradicating established biofilms.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary information.
References
Abedon ST (2016) Bacteriophage exploitation of bacterial biofilms: phage preference for less mature targets? FEMS Microbiol Lett 363: fnv246.
Agarwal R, Singh S, Bhilegaonkar K, Singh V (2011) Optimization of microtitre plate assay for the testing of biofilm formation ability in different Salmonella serotypes. Int Food Res J 18:1493
Ahiwale S, Tamboli N, Thorat K, Kulkarni R, Ackermann H, Kapadnis B (2011) In vitro management of hospital Pseudomonas aeruginosa biofilm using indigenous T7-like lytic phage. Curr Microbiol 62:335–340
Álvarez-Fernández E, Alonso-Calleja C, García-Fernández C, Capita R (2012) Prevalence and antimicrobial resistance of Salmonella serotypes isolated from poultry in Spain: comparison between 1993 and 2006. Int J Food Microbiol 153:281–287
Bedi MS, Verma V, Chhibber S (2009) Amoxicillin and specific bacteriophage can be used together for eradication of biofilm of Klebsiella pneumoniae B5055. World J Microbiol Biotechnol 25:1145
Bhowmick PP, Devegowda D, Ruwandeepika HD, Fuchs TM, Srikumar S, Karunasagar I, Karunasagar I (2011) gcpA (stm1987) is critical for cellulose production and biofilm formation on polystyrene surface by Salmonella enterica serovar Weltevreden in both high and low nutrient medium. Microb Pathog 50:114–122
Bielke L, Higgins S, Donoghue A, Donoghue D, Hargis B (2007) Salmonella host range of bacteriophages that infect multiple genera. Poult Sci 86:2536–2540
Borges KA, Furian TQ, Souza SN, Menezes R, Tondo EC, Salle CT, Moraes HL, Nascimento VP (2018) Biofilm formation capacity of Salmonella serotypes at different temperature conditions. Pesquisa Veterinaria Brasileira 38:71–76
Carson L, Gorman SP, Gilmore BF (2010) The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol Med Microbiol 59:447–455
Cerca N, Oliveira R, Azeredo J (2007) Susceptibility of Staphylococcus epidermidis planktonic cells and biofilms to the lytic action of Staphylococcus bacteriophage K. Lett Appl Microbiol 45:313–317
Chegini Z, Khoshbayan A, Moghadam MT, Farahani I, Jazireian P, Shariati A (2020) Bacteriophage therapy against Pseudomonas aeruginosa biofilms: a review. Ann Clin Microbiol Antimicrob 19:1–17
Chen S, Feng Z, Sun H, Zhang R, Qin T, Peng D (2021) Biofilm-formation-related Genes csgD and bcsA promote the vertical transmission of Salmonella Enteritidis in chicken. Front Vet Sci 7:625049
Cheong HJ, Lee YJ, Hwang IS, Kee SY, Cheong HW, Song JY, Kim JM, Park YH, Jung JH, Kim WJ (2007) Characteristics of non-typhoidal Salmonella isolates from human and broiler-chickens in southwestern Seoul, Korea. J Korean Med Sci 22:773–778
Chibeu A, Lingohr EJ, Masson L, Manges A, Harel J, Ackermann HW, Kropinski AM, Boerlin P (2012) Bacteriophages with the ability to degrade uropathogenic Escherichia coli biofilms. Viruses 4:471–487
Cornelissen A, Ceyssens PJ, T’syen J, Van Praet H, Noben JP, Shaburova OV, Krylov VN, Volckaert G, Lavigne R (2011) The T7-related Pseudomonas putida phage φ15 displays virion-associated biofilm degradation properties. PloS one 6:e18597
Cosby DE, Cox NA, Harrison MA, Wilson JL, Buhr RJ, Fedorka-Cray PJ (2015) Salmonella and antimicrobial resistance in broilers: a review. J Appl Poult Res 24:408–426
Dalmasso M, Strain R, Neve H, Franz CM, Cousin FJ, Ross RP, Hill C (2016) Three new Escherichia coli phages from the human gut show promising potential for phage therapy. PloS one 11:e0156773
Drescher SPM, Gallo SW, Ferreira PMA, Ferreira CAS, Oliveira SDD (2019) Salmonella enterica persister cells form unstable small colony variants after in vitro exposure to ciprofloxacin. Scientific Rep 9:1–11
Esmael A, Azab E, Gobouri AA, Nasr-Eldin MA, Moustafa M, Mohamed SA, Badr OA, Abdelatty AM (2021) Isolation and characterization of two lytic bacteriophages infecting a multi-drug resistant Salmonella Typhimurium and their efficacy to combat salmonellosis in ready-to-use foods. Microorg 9:423
Ferriol-González C, Domingo-Calap P (2020) Phages for biofilm removal. Antibiot 9:268
Fiorentin L, Vieira ND, Barioni JRW (2005) Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian pathol 34:258–263
García B, Latasa C, Solano C, Portillo FGD, Gamazo C, Lasa I (2004) Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol Microbiol 54:264–277
Garcia KCDOD, de Oliveira Corrêa IM, Pereira LQ, Silva TM, Mioni MdSR, de Moraes Izidoro AC, Bastos IHV, Gonçalves GAM, Okamoto AS, Andreatti Filho RL (2017) Bacteriophage use to control Salmonella biofilm on surfaces present in chicken slaughterhouses. Poult Sci 96:3392–3398
Gomez-Garcia J, Chavez-Carbajal A, Segundo-Arizmendi N, Baron-Pichardo MG, Mendoza-Elvira SE, Hernandez-Baltazar E, Hynes AP, Torres-Angeles O (2021) Efficacy of Salmonella bacteriophage S1 delivered and released by alginate beads in a chicken model of infection. Viruses 13:1932
Harms A, Fino C, Sørensen MA, Semsey S, Gerdes K (2017) Prophages and growth dynamics confound experimental results with antibiotic-tolerant persister cells. MBio 8:e01964-01917
Harper DR, Parracho HM, Walker J, Sharp R, Hughes G, Werthén M, Lehman S, Morales S (2014) Bacteriophages and biofilms. Antibiot 3:270–284
Hosny RA, Gaber AF, Sorour HK (2021) Bacteriophage mediated control of necrotic enteritis caused by C. perfringens in broiler chickens. Vet Res Commun 45:409–421
Hosseinidoust Z, Tufenkji N, van de Ven TG (2013) Formation of biofilms under phage predation: considerations concerning a biofilm increase. Biofouling 29:457–468
ISO6579-1 (2017) Microbiology of the food chain—horizontal method for the detection, enumeration and serotyping of Salmonella—part 1: detection of Salmonella spp: International Organization for Standardization Geneva, Switzerland.
ISO6579-3 (2014) Microbiology of the food chain—horizontal method for the detection, enumeration and serotyping of Salmonella—part 3: Guidelines for serotyping of Salmonella spp. International Organization for Standardization, Geneve.
Jain S, Chen J (2007) Attachment and biofilm formation by various serotypes of Salmonella as influenced by cellulose production and thin aggregative fimbriae biosynthesis. J Food prot 70:2473–2479
Kelly D, McAuliffe O, Ross R, Coffey A (2012) Prevention of Staphylococcus aureus biofilm formation and reduction in established biofilm density using a combination of phage K and modified derivatives. Lett Appl Microbiol 54:286–291
Khan AS, Georges K, Rahaman S, Abdela W, Adesiyun AA (2018) Prevalence and serotypes of Salmonella spp on chickens sold at retail outlets in Trinidad. PLoS One 13:e0202108
Kim MS, Lim TH, Jang JH, Lee DH, Kim BY, Kwon JH, Choi SW, Noh JY, Hong YH, Lee SB (2012) Prevalence and antimicrobial resistance of Salmonella species isolated from chicken meats produced by different integrated broiler operations in Korea. Poult Sci 91:2370–2375
Kirketerp-Møller K, Jensen PØ, Fazli M, Madsen KG, Pedersen J, Moser C, Tolker-Nielsen T, Høiby N, Givskov M, Bjarnsholt T (2008) Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol 46:2717–2722
Lau G, Sieo C, Tan W, Hair-Bejo M, Jalila A, Ho Y (2010) Efficacy of a bacteriophage isolated from chickens as a therapeutic agent for colibacillosis in broiler chickens. Poult Sci 89:2589–2596
Li X, He Y, Wang Z, Wei J, Hu T, Si J, Tao G, Zhang L, Xie L, Abdalla AE (2021) A combination therapy of phages and antibiotics: two is better than one. Int J Biol Sci 17:3573
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Mahmoud M, Askora A, Barakat AB, Rabie OEF, Hassan SE (2018) Isolation and characterization of polyvalent bacteriophages infecting multi drug resistant Salmonella serovars isolated from broilers in Egypt. Int J Food Microbiol 266:8–13
Manohar P, Tamhankar AJ, Lundborg CS, Nachimuthu R (2019) Therapeutic characterization and efficacy of bacteriophage cocktails infecting Escherichia coli, Klebsiella pneumoniae, and Enterobacter species. Front Microbiol 10:574
Mapes AC, Trautner BW, Liao KS, Ramig RF (2016) Development of expanded host range phage active on biofilms of multi-drug resistant Pseudomonas aeruginosa. Bacteriophage 6:e1096995
Merino L, Procura F, Trejo FM, Bueno DJ, Golowczyc MA (2019) Biofilm formation by Salmonella sp. in the poultry industry: detection, control and eradication strategies. Food Res Int 119:530–540
Nabil NM, Tawakol MM, Hassan HM (2018) Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect Ecol Epidemiol 8:1539056
Nabil NM and Yonis AEJAJFVS (2019) Isolation of Salmonella characterized by biofilm formation and disinfectant resistance from broiler chickens. Alex J Vet Sci 62.
Paluch E, Rewak-Soroczyńska J, Jędrusik I, Mazurkiewicz E, Jermakow K (2020) Prevention of biofilm formation by quorum quenching. Appl Microbiology Biotechnol 104:1871–1881
Pelyuntha W, Ngasaman R, Yingkajorn M, Chukiatsiri K, Benjakul S and Vongkamjan K (2021) Isolation and characterization of potential Salmonella phages targeting multidrug-resistant and major serovars of Salmonella derived from broiler production chain in Thailand. Front Microbiol 12.
Pires D, Sillankorva S, Faustino A, Azeredo J (2011) Use of newly isolated phages for control of Pseudomonas aeruginosa PAO1 and ATCC 10145 biofilms. Res Microbiol 162:798–806
Rahaman M, Rahman M, Rahman M, Khan M, Hossen M, Parvej M. and Ahmed S (2014) Poultry Salmonella specific bacteriophage isolation and characterization. Bangladesh J Vet Med 12.
Shang K, Wei B, Kang M (2018) Distribution and dissemination of antimicrobial-resistant Salmonella in broiler farms with or without enrofloxacin use. BMC Vet Res 14:257
Sorour HK, Gaber AF, Hosny RA (2020) Evaluation of the efficiency of using Salmonella Kentucky and Escherichia coli O119 bacteriophages in the treatment and prevention of salmonellosis and colibacillosis in broiler chickens. Lett Appl Microbiol 71:345–350
Spoering AL, Lewis K (2001) Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J bacteriol 183:6746–6751
Stepanović S, Ćirković I, Ranin L, S✓ vabić-Vlahović M, (2004) Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett Appl Microbiol 38:428–432
Tondo EC, Machado TRM, Malheiros PDS, Padrão DK, Carvalho ALD, Brandelli A (2010) Adhesion and biocides inactivation of Salmonella on stainless steel and polyethylene. Braz J Microbiol 41:1027–1037
Vongkamjan K, Benjakul S, Vu HTK, Vuddhakul V (2017) Longitudinal monitoring of Listeria monocytogenes and Listeria phages in seafood processing environments in Thailand. Food microbiol 66:11–19
Wang H, Ye K, Wei X, Cao J, Xu X, Zhou G (2013) Occurrence, antimicrobial resistance and biofilm formation of Salmonella isolates from a chicken slaughter plant in China. Food Control 33:378–384
Yang X, Brisbin J, Yu H, Wang Q, Yin F, Zhang Y, Sabour P, Sharif S, Gong J (2014) Selected lactic acid-producing bacterial isolates with the capacity to reduce Salmonella translocation and virulence gene expression in chickens. PLoS One 9:e93022
Zadeh RG, Kalani BS, Ari MM, Talebi M, Razavi S, Jazi FM (2022) Isolation of persistent cells within the biofilm and relative gene expression analysis of type II toxin/antitoxin system in Pseudomonas aeruginosa isolates in exponential and stationary phases. J glob antimicrob resist 28:30–37
Acknowledgements
The authors would like to thank Dr. Neveen Rabie for her help in the statistical analysis in the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Reham A. Hosny and Hend K. Sorour isolated S. enterica isolates, performed amplification and host range assay for Salmonella phage, examined the biofilm formation capability of S. enterica isolates, studied the antibiofilm activity of the phage against established biofilms produced by S. enterica isolates using microtiter plate assay. Azhar G. Shalaby performed the molecular detection of the biofilm-associated genes in S. enterica isolates and studied the antibiofilm activity of the phage against established biofilms produced by S. enterica isolates using reverse transcriptase real-time PCR. Reham A. Hosny, Azhar G. Shalaby, Soad A. Nasef, and Hend K Sorour wrote and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Consent to participate
All authors read and approved the manuscript.
Consent for publication
All authors read and approved the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hosny, R.A., Shalaby, A.G., Nasef, S.A. et al. Antibiofilm activity of a lytic Salmonella phage on different Salmonella enterica serovars isolated from broiler farms. Int Microbiol 26, 205–217 (2023). https://doi.org/10.1007/s10123-022-00294-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-022-00294-1