Abstract
Salmonella is a primary enteric pathogen related to the contamination of poultry and other food products in numerous foodborne outbreaks. The continuous emergence of multidrug-resistant bacteria has become a serious issue due to the overuse of antibiotics. Hence, lytic phages are considered alternative biocontrol agents against these bacterial superbugs. Here, two Salmonella phages—S4lw and D5lw—were subjected to genomic and biological characterization and further encapsulated to improve the stability under acidic conditions mimicking gastrointestinal conditions. The two lytic phages, S4lw and D5lw, taxonomically belong to new species under the Guernseyvirinae and Ackermannviridae families, respectively. Each phage showed antimicrobial activities against diverse Salmonella spp., such as S. Enteritidis and S. Typhimurium, achieving 1.7–3.4 log reduction after 2–6 h of treatment. The phage cocktail at a multiplicity of infection (MOI) of 100 or 1000 completely inhibited these Salmonella strains for at least 14 h at 25 °C. Additionally, the bead-encapsulated phage cocktail could withstand low pH and different simulated gut environments for at least 1 h. Overall, the newly isolated phages can potentially mitigate Salmonella spp. under the gastrointestinal environments through encapsulation and may be further applied via oral administration to resolve common antimicrobial resistance issues in the poultry production chain.
Similar content being viewed by others
Introduction
The poultry industry has grown drastically worldwide due to the increasing demand for the consumption of poultry meat and other related food products. The profit of low-cost production and high consumer demand have greatly driven the industry to produce nearly 100 million tons of poultry food products per year1. However, the pervasiveness of several common pathogens, including Salmonella, Listeria, and Campylobacter, has become a severe concern in the poultry industry2. In particular, Salmonella is widely detected in the poultry gastrointestinal tract, posing potential food safety risks to human health, such as Salmonellosis3. Two serovars of Salmonella from poultry origins were the primary cause of the human infection, of which 26% were of Salmonella enterica Enteritidis (S. Enteritidis), and 22% were of Salmonella enterica Typhimurium (S. Typhimurium)4. Salmonella infections can be elicited by direct human-to-poultry interaction, contamination of animal waste, and, most importantly, contaminated food. The continuous emergence of foodborne pathogens and their cross-contamination of food products during the processing pipeline in poultry facilities could result in a serious issue that requires extra effort.
Although Salmonella is one of the preeminent agents constantly contributing to foodborne illnesses in the United States, its capability to develop antibiotic resistance renders a greater threat to public health. The overuse and misuse of antibiotics in poultry have been the leading factors contributing to the increased antibiotic resistance in livestock. It has been reported that antibiotic-resistant Salmonella strains were commonly isolated from chicken meat around the world5. In the poultry production chain worldwide, such as poultry farms, layer farms, chickens in processing plants, and supermarkets, the presence of Salmonella was detected, with the highest resistance levels to nalidixic acid and ampicillin5. Moreover, due to the prevalence of antibiotic-resistant Salmonella, children, seniors, and immunosuppressed populations are much more susceptible to fatal infections6. The inability to use antibiotics as a standard treatment for those with Salmonellosis presents a future food safety threat to the public.
Bacteriophages (or phages) are viruses that infect bacteria. Based on the phage lifecycle, lytic phages can lyse their target bacterial host as natural predators. Therefore, lytic bacteriophages have been a promising antimicrobial agent for combating bacterial pathogens in the food industry. For example, several commercial phage products against foodborne pathogens—Listeria monocytogenes, Salmonella spp., and E. coli O157:H7—have been approved for application in food or production environments7. The ability of phages to explicitly target specific bacteria without harming the background microflora and host animal has been a highly desirable approach to treating Salmonella contamination. Additionally, the combination of diverse phages was vastly effective in eliminating target bacteria compared to individual phages. Several studies have investigated the antimicrobial activity of phage cocktails against Salmonella in vitro and in vivo. A previous study used a phage cocktail (CNPSA1, CNPSA3, and CNPSA4) to treat broiler chickens infected with S. Enteritidis PT4; phage-treated chickens had a 3.5-fold reduction in Salmonella cecal count compared to the control group8. In addition, several studies have focused on phage oral administration in chickens to improve the treatment effectiveness9,10. In most cases, phages were added to animal feeds or drinking water to enable effective oral administration into the chicken GI tract to control bacterial pathogens. One study indicated that a decreasing mortality rate of chickens and the absence of S. Typhimurium and S. Enteritidis in the chick cecum were observed after five successive doses of orally administered phages11. Most of all, using phages in chicken feed and water did not alter the chicken’s health or behavior, ensuring that phages are not toxic to host organisms nor affect their characteristics12. To diversify the library of Salmonella phages, this study characterized two newly isolated Salmonella phages—S4lw and D5lw—using genomic and biological approaches. In addition, the encapsulation of a two-phage cocktail (S4lw + D5lw) was further conducted to estimate their stability to sustain acidic stress in different simulated poultry gastrointestinal environments for controlling Salmonella contamination via oral administration.
Results
Phage genomic features
Phage S4lw contained double-stranded DNA with a 42,250-bp genome size and an average GC content of 49.7%. Sixty-nine coding DNA sequences (CDSs) were annotated in the genome of S4lw, 50 of which were predicted with the functions related to phage structure, DNA replication and repair, and host bacterial recognition and lysis (Fig. 1a). No virulence, antibiotic resistance, or lysogenic genes were detected in this phage genome. In addition, the genetic taxonomy results indicated that phage S4lw belonged to the Jerseyvirus genus under the Guernseyvirinae family. The phylogenetic tree showed that phage S4lw shared a close evolutionary relationship with Salmonella phage SETP7 (NC_022754) and Salmonella phage SETP13 (NC_022752) (Fig. 2a). In addition, the CDSs related to the crucial phage biological features, including bacterial host recognition (tail fiber, tail spike), host cell lysis (holin, endolysin), and phage replication (terminase large subunit), were genomically compared with the counterpart of other reference phages (Fig. 3a). The comparative genomics revealed that phage S4lw contained unique functional CDSs coding for tail fiber protein, holin, endolysin, and the terminase large subunit compared to the reference phages. Only the CDS encoding for tail spike protein in the phage S4lw shared a high nucleotide sequence similarity with the counterpart of Salmonella phage SE-W109.
Phylogenetic analysis of phages S4lw (a) and D5lw (b) constructed with the closely related phages using ViPTree database based on the alignment of phage complete genomes. The red stars indicate where phages S4lw and D5lw are positioned outside the trees for easy visualization. A zoom-in phylogenic tree is presented on the right panel of each phage containing the most closely related phage genomes to S4lw or D5lw. The viral family and bacterial host phylum classified by ViPTree are shown in the figure legend.
Phage D5lw had double-stranded DNA with a genome size of 157,399-bp and an average GC content of 44.7%. A total of 208 coding DNA sequences (CDSs) and 4 tRNAs (tRNA-Undet, tRNA-Ser, tRNA-Asn, and tRNA-Met) were found in the phage genome (Fig. 1b). There was no presence of harmful genes, such as virulence, antibiotic resistance, or lysogenic genes, in the phage D5lw genome. The genetic taxonomy indicated that phage D5lw was classified in the Kuttervirus genus under the Ackermannviridae family. D5lw was genomically similar to three Salmonella phages: Pertopsoe (NC_049507), Maane (NC_049508) and Allotira (NC_049501) (Fig. 2b). The CDSs related to critical biological traits were further compared with the counterparts of the close-related reference phages, as shown in Fig. 3b. The predicted CDSs coding for tail spike and tail fiber proteins in phage D5lw, related to phage infection, were closely related to that of Salmonella phage vB SenM UTK0004 and Salmonella phage Bering, respectively. The CDSs of lysin and holin, both associated with host lysis, in the D5lw genome showed a high nucleotide similarity to the counterparts in Salmonella phage Maane and Salmonella phage D3F6, respectively (Fig. 3b). Furthermore, phage D5lw contained the CDS of terminase large subunit, sharing a high nucleotide sequence similarity with Salmonella phage vB SalM SJ2, Salmonella phage vB SenA AR2819 and Escherichia phage Sa157lw (Fig. 3b).
Biological characterization of phages
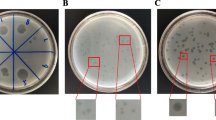
Phage S4lw had a capsid with approximately 64.5 ± 0.5 nm in diameter and a long non-contractile tail of 132.3 ± 0.5 nm in length (Fig. 4a). Phage D5lw had a capsid of approximately 96.7 ± 0.5 nm in diameter and a long contractile tail of 135.5 ± 0.5 nm in length (Fig. 4b). Both phages produced clear plaques on plaque-assay plates, indicating their lytic capability against Salmonella Typhimurium (Fig. 4c and d). Moreover, a distinct halo surrounding each lysis zone caused by phage S4lw was observed on the plate (Fig. 4c).
Transmission electron microscopy image of S4lw (a) and D5lw (b). The morphology of phage S4lw (a) has a capsid (64.5 ± 0.5 nm in diameter) and a long non-contractile tail (132.3 ± 0.5 nm in length), belonging to the Guernseyvirinae family. Phage D5lw (b) has a morphology containing a capsid (96.7 ± 0.5 nm in diameter) and a long contractile tail (135.5 ± 0.5 nm in length), belonging to the Ackermannviridae family. The plaque morphologies of S4lw (c) and D5lw (d) are shown on a plaque-assay plate against Salmonella Typhimurium ATCC14028; both phages produced clear plaques with an average size of 0.523 ± 0.01 mm and 0.517 ± 0.02 mm in diameter, respectively. A distinct halo around each lysis zone caused by phage S4lw was observed on the plates and indicated with a white arrow (c).
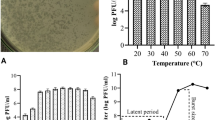
In addition, the one-step growth curve was conducted against Salmonella Typhimurium ATCC14028 for both phages to evaluate their growth factors. The results showed a complete lytic cycle for phage S4lw and D5lw was 65 min and 60 min, respectively (Fig. 5). Phage S4 had a latent period of 12 min with a burst size of 41 PFU per infected cell (Fig. 5a), while Phage D5lw performed a relatively shorter latent period of 4 min with a burst size of 37 per infected cell (Fig. 5b).
One-step growth curve of the phage S4lw (a) and D5lw (b) using Salmonella Typhimurium ATCC14028. The growth parameters of the phage indicate a latent period (LP) and an average burst size (BS) of each phage. The error bars present the standard error of the mean for each time point of the one-step growth curve.
Antimicrobial activity of two phages
Each Salmonella phage was able to infect a broad spectrum of Salmonella serovars, including S. Typhimurium, S. Enteritidis, S. Heidelburg, and S. Saintaual (Table 1). The bacterial challenge assay indicated that the two-phage cocktail (D5lw + S4lw) had the highest antimicrobial activity against bacterial host ATCC14028 than the individual phage S4lw and D5lw (data not shown). Therefore, the two-phage cocktail against different Salmonella strains at MOIs of 0.1, 1, 10, 100, and 1000 was further determined for biocontrol potential. The results showed that the phage cocktail with the MOI of 100 or 1000 completely inhibited each of the selected Salmonella strains for at least 14 h at 25 °C (Fig. 6). Moreover, S. Typhimurium ATCC14028, S. Enteritidis PT-30, and S. Saintpaul 39 were completely inhibited by the phage cocktail with the MOIs of 100 and 1000 for 16 h (Fig. 6a, c and d). In addition, the occurrence of lysis from without for each phage treatment group was determined. The results indicated that no lysis from without was observed on all five tested Salmonella strains treated with phage cocktail at the MOIs of 100 and 1,000 (data not shown), demonstrating the strong antimicrobial activities of our phage cocktail.
Bacterial challenge assay of a Salmonella phage cocktail (S4lw + D5lw) against (a) Salmonella Typhimurium ATCC14028, (b) Salmonella Heidelburg 45955, (c) Salmonella Saintpaul 39, (d) Salmonella Enteritidis PT-30, and (e) Salmonella Enteritidis H3527 with different MOIs. The OD600 was measured every hour for 16 h at 25 °C. The control contained bacterial culture only. The treatment groups are the mixture of bacterial culture and the phage cocktail with different MOIs of 0.1, 1, 10, 100 and 1000. Each color point is an average of three repeats, with standard error bars.
Additionally, during the treatment of S. Typhimurium (ATCC14028) by the phage cocktail, the results revealed that the phages significantly reduced the bacterial concentration and attained the highest reduction of 3.4 log after 6-h treatment in comparison to the control (Fig. 7a). A similar result was observed for the phage cocktail against a bacterial panel of five- Salmonella strains (Fig. 7b). The phage treatment group had significantly lower bacterial levels than the control group, causing a 1.7–2.2-log reduction after 4 to 6 h of the phage treatment.
Antimicrobial activities of a two-phage cocktail (S4lw + D5lw) against Salmonella Typhimurium ATCC14028 (a) and a five-strain bacterial panel (b) at an MOI of 100 in TSB at 25 °C for 24 h. The control group contained bacterial culture only. The phage treatment group contained bacterial culture treated with the phage cocktail. Each column represents an average of three repeats, with standard error bars. Data of each time point (0, 2, 4, 6, 24 h) were analyzed separately, and an asterisk indicates significant differences between the control and phage treatment groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Phage encapsulation and in vitro stability test in acid environments
To enhance our phage stability against Salmonella spp. under various acid stresses, the phage cocktail was encapsulated in sodium alginate with different sizes of beads (Supplementary Table S1). The results showed that a 200-µm nozzle size produced the smallest phage-encapsulated beads, with an average of 0.219 mm and the highest encapsulation efficacy of 4.4%, among the 300-, 450-, 750-, and 1000-µm nozzle sizes. Therefore, the encapsulated phages from the 200-µm nozzle size were used for the downstream analysis.
pH ranging from 2 to 7 was chosen for the pH stability test on free phages and the encapsulated phage cocktail at 42 °C, mimicking the gastrointestinal tract (GIT) temperature of chickens (Fig. 8a). The result disclosed both free phages S4lw and D5lw were below the detection level at pH 2; however, the encapsulated phages with around 2.58 log10 PFU/mL (0.05%) were recovered after the treatment at pH 2 for one hour. In addition, around 5% free phage S4lw (around 6.67 log10 PFU/mL) was detected at pH 3 and 20–30% of the phage survived during pH 4–7. However, free phage D5lw had an average of 0.07% survival at pH 3 and 20% survival rate from pH 4 to 7 under the same condition. Additionally, most encapsulated phages maintained a similar titer before and after treatment from pH 3 to 7 at 42 °C.
pH test of free phages S4lw, D5lw, and encapsulated phage cocktail produced by a 200-µm nozzle size. (a) The phages were added to the SM buffer with different pH levels (pH2, pH3, pH4, pH5, pH6, and pH7) at 42 °C for one hour before neutralization and the survival phage determination. (b) The phages were added to the different gastrointestinal tract (GIT)-related conditions (simulated gastric fluid (SGF), bile salt, and simulated intestinal fluid (SIF)) at 42 °C for one hour before neutralization and the survival phage determination. Each column represents an average of three repeats, with standard error bars. Data of each pH level were analyzed separately, and the asterisk indicates significant differences among phages S4lw, D5lw, and encapsulated phage cocktail. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Both free and encapsulated phages were used for the stability test in the simulated GIT fluids at 42 °C (Fig. 8b). The result showed that both free and encapsulated phages were inactivated in the simulated gastric fluid (SGF) at pH 2.4. Free phages S4lw and D5lw demonstrated a low survival rate of 17.9% and 2.5% in a simulated bile salt solution, respectively, whereas 64% of the encapsulated phages were detected. Similar results were observed in the simulated intestinal fluid (SIF) for free phages (23.27% for phage S4lw and 2% for phage D5lw) and encapsulated phage cocktail (54%). These results indicated that encapsulation rendered additional protection for free phages to sustain the gastrointestinal stresses.
Discussion
In this study, two phages—S4lw and D5lw—were isolated from environmental samples and characterized as the potential natural predators against various pathogenic Salmonella spp. As the most abundant biological entities in the biosphere, a vast number of phages remain to be explored. The taxonomy guideline by the International Committee on Taxonomy of Virus (ICTV) indicates that two phages can be assigned to the same species if their genome identical (coverage x identity) exceeds 95% at the nucleotide level over the full genome length by use of genomic tools, such as BLASTn. The BLASTn result in the current study showed that phage S4lw shared the highest nucleotide sequence similarity (84.17%) to Salmonella phage SGPC (OK169616) (coverage of 91% and identity of 92.50%), while phage D5lw shared the highest nucleotide identical of 88.96% (coverage of 91% and identity of 97.76%) with Salmonella phage moki (NC_049506). The comparative genomics also confirmed that phages S4lw and D5lw were not classified to any known species under the Jerseyvirus genus and verified species under Genus Kuttervirus, respectively (data not shown). As a result, phages S4lw and D5lw are likely proposed as two new species under the respective genera of Jerseyvirus and Kuttervirus, contributing to the diversity of the phage pool.
Phage stability is a critical factor ensuring the efficiency of phage-based treatment or phage therapy after administration. Temperature and pH are two of the common environmental factors that influence phage stability. To evaluate the effectiveness of our phage cocktail against Salmonella in a chicken GIT environment, low pH values with 42 °C were used to test phage stability in this study. The acidic test results indicated that free phage D5lw was not detected at pH 2 and 3; thus, a protection method was necessary to enhance the stability for oral delivery of the phage. Several hydrogel-based materials, including whey protein isolate (WIP), Eudragit S100, and chitosan, have been applied by encapsulation for phage oral delivery13,14. In the current study, sodium alginate was used for phage encapsulation due to the industry's low production cost of potetial application. In contrast to free phages, the stability results indicated that the encapsulation process did improve the phage survival rate at pH 3–7 and in the simulated intestinal system. However, the results showed that both free and encapsulated phages were completely inactivated in the SGF at pH 2.4 after 1-h incubation at 42 °C. Our finding was consistent with a previous study that free phages were rapidly inactivated in 20 min at pH 2.0, and encapsulated phages also reached a complete inactivation after 60-min treatment at pH 2.015. Chicken gizzard has the lowest pH environment around pH 2–3 compared to other GIT locations16. To overcome the low-pH challenge in chicken gizzards, where food usually stays for 45–60 min16,17, the mixture of encapsulated phages and feed may improve the phage survival rate and allow some phages to be delivered to the intestines. A previous study incorporated encapsulated phages in animal feed pellets to test the stability of encapsulated phages after extruding and acid exposure18. Their results indicated that alginate-only capsules had no phage loss following extrusion during pellet production and showed a better phage survival rate after acid exposure than the control group. These findings provide a solution regarding oral delivery of phages by adding the encapsulated phages into animal feed. More studies regarding the stability of our encapsulated phages during the pellet production process and acid exposure are necessary.
Overall, two new lytic phages, S4lw and D5lw, were isolated and characterized for controlling Salmonella in different conditions. These two phages have no harmful genes in their genomes and are safe for phage application. Both phages have a broad lysis spectrum and can render strong lytic activities against various Salmonella serovars. Additionally, alginate was used to encapsulate the two-phage cocktail and provided extra protection for the phages to sustain acidic stress in simulated gut environments. The findings of this study demonstrate that phages S4lw and D5lw are promising biocontrol agents and could be further developed for oral phage therapy to combat Salmonella pathogens in poultry gut environments. Future studies regarding the treatment effectiveness and safety of our Salmonella phage cocktail against Salmonella pathogens in poultry via oral delivery application are required.
Methods
Bacteria culture
A collection of the Salmonella enterica strains were obtained from the Produce Safety and Microbiology (PSM) Research Unit at the U.S. Department of Agriculture (USDA), Agricultural Research Service (ARS), Western Regional Research Center (WRRC), Albany, CA, United States for this study (Table 1). Salmonella Typhimurium ATCC14028 was used for phage propagation and quantification. Fresh bacterial culture was prepared by inoculating 10 ml TSB with 1 μl loopful of each strain for overnight incubation at 37 °C before use.
Phage isolation
Salmonella phages S4lw and D5lw were isolated from sewage water collected from the University of California, Davis sewage plant using the bacterial host of Salmonella Typhimurium ATCC14028. Two phages—S4lw and D5lw—were purified using the single-plaque purification method and further propagated with overnight ATCC14028 culture and 10 mM CaCl2 in 40 mL of tryptic soy broth (TSB; Difco, Becton, Dickinson, Sparks, MD USA) at 37 °C for 18 h. The enriched phages were centrifuged at 8000 × g for 10 min at 4 °C and filtered through a 0.22-μm filter membrane to remove bacterial debris. The obtained phage lysates were concentrated via a 50 kDa cutoff Amicon Ultra-15 Centrifugal Filter Unit (Merck Millipore, Billerica, MA, USA) and purified by CsCl gradient ultracentrifugation as previously described19.
Whole genome sequencing and genomic analysis of phage S4lw and D5lw
The DNA of phage S4lw and D5lw was extracted using a Norgen Biotek phage DNA extraction kit (Thorold, ON, Canada) following the manufacturer’s instructions. The whole genome sequencing was conducted as previously described20. Briefly, the DNA library of two phages was constructed using a TruSeq Nano DNA library prep kit (Illumina, San Diego, CA) with the 500 bp sequence length and then sequenced on an Illumina MiSeq sequencer (Illumina, San Diego, CA, USA) based on the manufacturer’s instructions. A total of 12,529,574 and 6,694,250 sequence reads were generated for phages S4lw and D5lw, respectively. The phage genome assembly and annotation were processed as previously described21. First, the high-quality reads were obtained using FASTQC and Trimmomatic with the setting of Q30 and then subjected to the de novo assembly using Megahit (version 1.2.9). Genome annotation was performed using RAST (Version 2.0) and Prokka (Version 1.14.5) with the default parameters. The final annotation of two phages was confirmed by manual correction using Geneious (version 2023.02, Biomatters, New Zealand) based on BLASTp results against the Uniprot database, with the nucleotide similarity above 90%. The screening of tRNA, virulence genes, and antibiotic resistance genes in the phage genomes was performed via tRNAscan-SE v.2.022, VirulenceFinder v2.0 (https://cge.cbs.dtu.dk/services/VirulenceFinder/; accessed on 02/01/2023)23, and ResFinder v4.1 (https://cge.cbs.dtu.dk/services/ResFinder/; accessed on 02/01/2023)24, respectively.
The complete genomes of phages S4lw and D5lw were subjected to taxonomic classification and phylogenetic analysis with other closely related reference phage genomes using BLASTn and ViPTree version 3.425, respectively. The functional genes related to phage replication, bacterial host recognition, and host cell lysis were extracted and subsequently compared with the counterparts of the reference phage genomes via MEGA11 with the ClustalW algorithm and Maximum likelihood tree26.
Phage morphology
The morphologies of Salmonella phages S4lw and D5lw were observed using transmission electron microscopy (TEM) (Tecnai G2 F20 model FEI, USA) as previously described19.
One-step growth curve
One-step growth curve experiments were conducted as previously described with subtle modification19. Phage (S4lw or D5lw) was added to the bacterial culture of S. Typhimurium ATCC14028, with a multiplicity of infection (MOI) of 0.01, and incubated at 37 °C (10 min) for phage adsorption. After centrifuging at 5000 × g for 5 min at 4 °C, the bacterial pellet was washed three times with 2 mL of TSB and subsequently resuspended in 20 mL TSB. The resuspended culture was further 100-fold diluted in 30 mL TSB and incubated at 37 °C with shaking at 90 rpm throughout the entire experiment. Upon culture resuspension, phage-infected bacterial cell counts were determined by mixing 50 μL of the sample (diluted culture) with 100 μL of the overnight culture of S. Typhimurium ATCC14028 and 5 mL of molten 50% TSA agar prior to pouring into a pre-poured 15-mL TSA plate. During the incubation, 1 mL of sample was collected at a 10-min interval for phage D4lw and S4lw for a total of 80 min. The sample from each time point was filtered through a sterile 0.22-μm membrane filter for double-layer plaque assay to determine phage latent periods and burst sizes. The one-step growth curve experiment of each phage was performed three times.
Determination of phage host range
Phages S4lw and D5lw were tested for their host range against nine different serovars of Salmonella strains (Table 1) using the spot test assay as previously described19. Briefly, 5 μl of each tenfold diluted phage lysate (6–9 log PFU/mL) were spotted on the TSA plates pre-mixed with individual bacterial cultures (around 8 log CFU) from the selected host panel in 5 ml molten 50% TSA on the top layer. The level of clearing on the spotted area was observed to evaluate the phage lysis ability against different strains.
Antimicrobial activity of phage cocktail against Salmonella
The bacterial challenge assay of a two-phage cocktail (S4lw and D5lw with the ratio of 1:1) against different Salmonella strains with different MOIs was determined using a spectrophotometer (Promega, Madison, WI, USA). In brief, 180 μl per well of the diluted bacterial culture at the concentration of 1 × 105 CFU/ml was dispensed to a 96-well plate. Subsequently, an aliquot of 20 μl of phage cocktail per well was added to reach the MOIs of 0.1, 1, 10, 100, and 1000 accordingly. Twenty microliters of SM buffer without phage were added as the control. Both control and phage treatment groups with each MOI had three replications. The optical density at 600 nm (OD600) reading was recorded every 5 min for the first 1 h to determine the bacterial lysis without complete phage infection (lysis from without) and every hour for 16 h to access the antimicrobial activity of the phage cocktail at room temperature (25 °C).
In addition, the antimicrobial activities of two phages against either a single bacterial strain of ATCC14028 or a panel of five susceptible Salmonella strains (S. Typhimurium ATCC14028, S. Enteritidis PT-30, S. Enteritidis H3527, S. Saintpaul 39, S. Heidelburg 45955) were determined as previously described27. The overnight bacterial culture was diluted in 40 mL of LB broth (Invitrogen, Carlsbad, CA, USA) to reach the final concentration of 1 × 105 CFU/ml. The phage cocktail was added to the bacterial solution with an MOI of 100 as the treatment group, while the control group was added with the same volume of SM buffer. The control and treatment groups were incubated at 25 °C for 24 h. Meanwhile, the bacterial concentration was determined at 0, 2, 4, 6, and 24 h by spread plating on XLD (BD, Franklin Lakes, NJ) with thin TSA overlay (BD, Franklin Lakes, NJ) (Thin Agar Layer Method, TAL)28.
Encapsulation of a Salmonella two-phage cocktail
The encapsulation of a phage cocktail—S4lw and D5lw—was conducted as previously described with minor modification29. First, phages S4lw and D5lw at approximately 109 PFU/mL were mixed together at a 1:1 ratio as a phage cocktail. A total of 100 mL of phage cocktail in SM buffer was added with 0.02 M Na2CO3 (Sigma-Aldrich, Oakville, Ontario, Canada) and around 1.2—1.8% sodium alginate (Buchi, Switzerland), depending on nozzle sizes used for the encapsulation. After alginate was dissolved, the phage-alginate mixture was subject to encapsulation using a Buchi B-390 encapsulator (Buchi, Switzerland) with different nozzle sizes (200, 300, 450, 750, and 1000 μm) accordingly. At the same time, a clean beaker containing 200 mL of 1.8% CaCl2 (Sigma-Aldrich, Oakville, Ontario, Canada) with a low-rate spinning stir bar was used to catch and harden the resulting phage-encapsulated beads from the encapsulator. After solidifying, the beads were separated from the solution and washed with SM buffer (three times) to remove any free phages. The beads were stored in the SM buffer at 4 °C for further experiments.
Efficiency of phage cocktail encapsulation using different nozzle sizes
The encapsulation efficacy of phages was determined by measuring the phage concentration encapsulated within the beads as previously described29. A total of 1 mL phage-encapsulated beads was added to 9 ml of microsphere-broken solution (MBS) to dissolve the alginate layer and release the phages. A double-layer plaque assay method was used to determine the initial concentration of phages used for encapsulation and the phages released from the encapsulated beads. The encapsulation efficiency (EE) was calculated as EE% = (phage concentration released from the dissolved microspheres /initial phage concentration used for encapsulation) × 100%.
Acid test of free and encapsulated phages
The pH stability tests, ranging from pH 2 to pH 7, were conducted on free phages S4lw, D5lw, and the encapsulated phage cocktail as previously described with subtle revision27. Briefly, 100 μl of single phage S4lw, D5lw, or encapsulated phage cocktail (around 6 log10 PFU/mL) was added to 9.9 ml of SM buffer with the final pH values of 2, 3, 4, 5, 6, and 7. The samples were incubated at 42 °C for 1 h before phage quantification via a double-layer plaque assay. For the encapsulated phage, the acid-treated phage beads were dissolved in MBS to release the phage prior to the double-layer plaque assay to determine the stability.
Phage stability in simulated gastric fluid, bile salt, and simulated intestinal fluid
The stability of free individual phages and the encapsulated phage cocktail was tested under different simulated GIT conditions as previously described30. In detail, SGF consisted 3.2 mg/ml pepsin (Sigma-Aldrich, Oakville, Ontario, Canada) in 0.2% (wt/vol) NaCl at pH 2.4. Bile salt contained 1% or 2% (wt/vol) porcine bile extract (Sigma-Aldrich, Oakville, ON, Canada). Simulated intestinal fluid (SIF) had 10 mg/ml pancreatin (Sigma-Aldrich, Oakville, Ontario, Canada) in 50 mM KH2PO4 at pH 6.830. An aliquot of 100 μl free or encapsulated phages (around 6 log10 PFU/mL) was added to 9.9 mL of the solutions (SGF, bile salt, and SIF) at 42 °C for 1 h to determine the phage stability. After 1-h treatment, the survival phage concentration was obtained using the method described in the pH test section.
Statistical analysis
Experiments were conducted in three individual repeats. Two-way analysis of variance (ANOVA) with Šídák’s multiple comparisons test was used to evaluate the effects of lysis from without, bacterial log reduction, and phage stability tests at a statistical significance of 5% via GraphPad Prism 10.
Data availability
The complete genome sequences of Salmonella phage S4lw and D5lw have been deposited in GenBank under the accession numbers OQ660438 and OQ660437, respectively.
References
Islam, Md. R. et al. A systematic review from basics to omics on bacteriophage applications in poultry production and processing. Crit. Rev. Food Sci. Nutr. 0, 1–33 (2021).
Ayoola, M. B., Pillai, N., Nanduri, B., Rothrock, M. J. & Ramkumar, M. Preharvest environmental and management drivers of multidrug resistance in major bacterial zoonotic pathogens in pastured poultry flocks. Microorganisms 10, 1703 (2022).
Andino, A. & Hanning, I. Salmonella enterica: survival, colonization, and virulence differences among serovars. Sci. World J. 2015, e520179 (2015).
Shivaning Karabasanavar, N. et al. Prevalence of Salmonella serotypes S. Enteritidis and S. Typhimurium in poultry and poultry products. J. Food Saf. 40, e12852 (2020).
Castro-Vargas, R. E., Herrera-Sánchez, M. P., Rodríguez-Hernández, R. & Rondón-Barragán, I. S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet World 13, 2070–84 (2020).
Zwe, Y. H. et al. Prevalence, sequence types, antibiotic resistance and gyrA mutations of Salmonella isolated from retail fresh chicken meat in Singapore. Food Control 90, 233–240 (2018).
Housby, J. N. & Mann, N. H. Phage therapy. Drug Discov. Today 14, 536–540 (2009).
Fiorentin, L., Vieira, N. D. & Barioni, W. Jr. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. 34, 258–263 (2005).
Evran, S., Tayyarcan, E. K., Acar-Soykut, E. & Boyaci, I. H. Applications of Bacteriophage Cocktails to Reduce Salmonella Contamination in Poultry Farms. Food Environ Virol. 14, 1–9 (2022).
Żbikowska, K., Michalczuk, M. & Dolka, B. The use of bacteriophages in the poultry industry. Animals (Basel) 10, 872 (2020).
Nabil, N. M., Tawakol, M. M. & Hassan, H. M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 8, 1539056 (2018).
Clavijo, V. et al. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 98, 5054–5063 (2019).
Petsong, K., Benjakul, S. & Vongkamjan, K. Optimization of wall material for phage encapsulation via freeze-drying and antimicrobial efficacy of microencapsulated phage against Salmonella. J. Food Sci. Technol. 58, 1937–1946 (2021).
Yang, Y. et al. Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: A review. J. Controll. Releas. 353, 634–649 (2023).
Zhang, B. et al. Microencapsulated phage composites with increased gastrointestinal stability for the oral treatment of Salmonella colonization in chicken. Front Vet. Sci. 9, 1101872 (2023).
Gomez-Garcia, J. et al. Efficacy of Salmonella bacteriophage S1 delivered and released by alginate beads in a chicken model of infection. Viruses 13, 1932 (2021).
Amerah, A. M. & Ravindran, V. Chapter 3 - effect of whole wheat feeding on gut function and nutrient utilization in poultry. In Wheat and rice in disease prevention and health (eds Watson, R. R. et al.) 35–40 (Academic Press, 2014).
Richards, K. & Malik, D. J. Bacteriophage encapsulation in pH-responsive core-shell capsules as an animal feed additive. Viruses 13, 1131 (2021).
Zhang, Y., Liao, Y.-T., Salvador, A., Lavenburg, V. M. & Wu, V. C. H. Characterization of two new Shiga toxin-producing Escherichia coli O103-infecting phages isolated from an organic farm. Microorganisms 9, 1527 (2021).
Zhang, Y., Liao, Y.-T., Salvador, A., Sun, X. & Wu, V. C. H. Complete genome sequence of a Shiga toxin-converting bacteriophage, Escherichia phage Lys12581Vzw, induced from an outbreak Shiga toxin-producing Escherichia coli strain. Microbiol. Resour. Announc. 8, e00793-e819 (2019).
Zhang, Y., Liao, Y.-T., Salvador, A. & Wu, V. C. H. Genomic characterization of two Shiga toxin-converting bacteriophages induced from environmental Shiga toxin-producing Escherichia coli. Front. Microbiol. 12, 587696 (2021).
Chan, P. P., Lin, B. Y., Mak, A. J. & Lowe, T. M. tRNAscan-SE 2.0: Improved detection and functional classification of transfer RNA genes. Nucleic. Acids Res. 49, 9077–96 (2021).
Joensen, K. G. et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 52, 1501–1510 (2014).
Bortolaia, V. et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob. Chemother. 75, 3491–500 (2020).
Nishimura, Y. et al. ViPTree: The viral proteomic tree server. Bioinformatics 33, 2379–2380 (2017).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35, 1547–1549 (2018).
Liao, Y.-T., Zhang, Y., Salvador, A., Harden, L. A. & Wu, V. C. H. Characterization of a T4-like bacteriophage vB_EcoM-Sa45lw as a potential biocontrol agent for Shiga toxin-producing Escherichia coli O45 contaminated on mung bean seeds. Microbiol. Spectrum. 10, e02220-e2221 (2022).
Wu, V. C. H. A review of microbial injury and recovery methods in food. Food Microbiol. 25, 735–744 (2008).
Colom, J. et al. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy. Sci Rep. 7, 41441 (2017).
Ma, Y. et al. Microencapsulation of bacteriophage Felix O1 into Chitosan–Alginate microspheres for oral delivery. Appl. Environ. Microbiol. 74, 4799–4805 (2008).
Acknowledgements
This work was supported by the U.S. Department of Agriculture, Agricultural Research Service, under CRIS project number 2030-42000-055-000-D.
Author information
Authors and Affiliations
Contributions
Y.Z. performed bacterial challenge assay, lysis from without, data analyses, genomic analysis, and manuscript preparation. M.C. performed phage encapsulation and phage stability tests. Y.-T.L. performed phage isolation and manuscript revision. A.S. performed the whole-genome sequencing and manuscript revision. V.C.H.W. conceived and supervised the study, aided in experiment design, and edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Chu, M., Liao, YT. et al. Characterization of two novel Salmonella phages having biocontrol potential against Salmonella spp. in gastrointestinal conditions. Sci Rep 14, 12294 (2024). https://doi.org/10.1038/s41598-024-59502-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59502-9
- Springer Nature Limited