Abstract
Background
Gastric cancer often exhibits discrepancies between the gross and pathological tumor boundaries, and the degree of discrepancy may be a tumor characteristic. However, whether these discrepancies influence oncological outcomes remains unclear.
Methods
The data of patients who underwent total gastrectomy for gastric cancer from 2005 to 2018 were collected. A new parameter, ΔPM, which corresponds to the length of the discrepancy between the gross and pathological proximal boundaries, was calculated and the patients were divided into two groups: patients with long ΔPM and those with short ΔPM. Oncological outcomes were compared between the two groups.
Results
A length of 8 mm was determined as the cutoff value for long or short ΔPM. Tumor size, growth pattern, pathological type, depth, and esophageal invasion were associated with ΔPM > 8 mm. Overall survival of the ΔPM > 8 mm group was significantly worse than that of the ΔPM ≤ 8 mm group (5-year overall survival: 58% vs 78%; p < 0.0001). Multivariate analysis revealed that ΔPM > 8 mm was an independent risk factor for poor survival and peritoneal metastasis. The likelihood ratio test revealed a significant interaction between pT status and ΔPM (p = 0.0007). Circumferential involvement and gross esophageal invasion were poorer survival factors in the ΔPM > 8 mm group.
Conclusions
ΔPM > 8 mm is related to several clinicopathological characteristics and is an independent risk factor for poorer survival and peritoneal metastasis but not local recurrence. ΔPM > 8 mm combined with circumferential involvement or esophageal invasion is associated with relatively poor survival outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fifth most common cancer and the third leading cause of cancer death worldwide, with approximately 1,089,103 new patients and 768,793 deaths every year [1]. Complete resection is the only major curative treatment for most patients with this lethal disease [2, 3]. To achieve the long-term oncological safety of gastric cancer, it is essential to ensure that there is no residual tumor. Thus, maintaining a pathologically negative resection margin in gastrectomy is the minimum requirement necessary to achieve a cure.
Pathologically residual cancer, including positive resection margin, is associated with poor survival [4,5,6,7]. Gastric cancer often has discrepancies between the gross and pathological tumor boundaries. Thus, we usually transect the stomach or the esophagus in curative gastrectomy by considering some safety margins to achieve complete resection with no residual cancer. Previously, to determine recommended lengths to ensure a pathologically negative margin, we evaluated the length of discrepancy between the gross and pathological proximal boundaries of the tumor (ΔPM) in each type of gastric cancer or esophagogastric junction cancer [8,9,10]. Using this metric, we can obtain a pathologically negative margin when we transect the stomach or the esophagus by maintaining ΔPM beyond the tumor boundary.
In our previous studies, we found that the ΔPM could vary in each patient and almost represented a standard distribution. These previous studies also revealed that the maximum ΔPM, which was defined as a value that first became 0 in a number of patients towards the plus direction of the histogram, varied according to the gross tumor type, pathological type, or size of tumor size [8,9,10]. These findings indicate that the degree of ΔPM may be associated with one of these tumor characteristics. Furthermore, another study previously established by us revealed that patients whose pathological proximal margin (PM) was once positive but who underwent additional resection to achieve complete resection still had worse survival outcomes [11]. Tumors for which pathological PM was once positive for cancer might have a long ΔPM. Considering these facts, the degree of ΔPM may be associated with oncological outcomes. If this is true, we can refer the information to daily practice or establish a new therapeutic strategy according to the degree of ΔPM. However, no studies regarding this issue have been conducted.
In this study, to identify whether the ΔPM we had newly defined as unexpected horizontal tumor spread has oncological impact, we retrospectively evaluated survival outcomes according to the ΔPM of gastric cancer that requires total gastrectomy.
Patients and methods
Patient selection
In this retrospective study, data were retrieved from our prospectively developed database. We collected consecutive patients who underwent total gastrectomy for gastric cancer, gastric cancer with esophageal invasion (EI), or Siewert type III esophagogastric junction cancer at the Cancer Institute Hospital, Tokyo, Japan, between January 2005 and December 2018. In this study, we limitedly collected patients who underwent total gastrectomy to match the tumor location and the surgical procedure, which influence survival outcomes. We excluded patients who had undergone endoscopic submucosal dissection, received neoadjuvant chemotherapy, had Siewert type I or II disease, underwent R1/R2 resection, or had missing clinical data (Supplementary Fig. 1).
Patients with cT1 disease were classified into the superficial growth type (Sup), and those with cT2–4 disease were divided into two groups based on their preoperative examination findings: expansive growth (Exp) or infiltrative growth type (Inf). The classification basically fits the gross type of tumor defined by the Japanese Classification of Gastric Carcinoma (JCGC), 3rd English Edition [12]; that is, type 1 and 2 tumors were classified as Exp, and type 3 and 4 tumors were classified as Inf. In patients with type 5 tumors, the findings of preoperative examinations were re-evaluated, and the tumors were then classified as Exp or Inf. In addition, patients were divided into two groups on the basis of their postoperative histopathological findings: differentiated and undifferentiated types (Dif and Und). Dif contained well or moderately differentiated adenocarcinoma and papillary adenocarcinoma, while Und contained solid or non-solid types of poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma according to the JCGC [12]. The clinical tumor status was also determined based on the JCGC [12] using endoscopy and enhanced computed tomography findings. This study was approved by the Institutional Review Board of the Cancer Institutional Hospital (IRB No. 2020–1270). All the patients were fully informed and provided consent through an opt-out approach.
Surgical procedure and measurement of gross PM length
All patients enrolled in the present study underwent total gastrectomy with D1+ lymphadenectomy for cT1 disease or D2 lymphadenectomy for cT2–4 or cN-positive disease according to the Japanese Gastric Cancer Treatment Guidelines [13].
As we previously reported [8,9,10], fresh specimens were opened longitudinally, and the lymph nodes were removed for pathological examination; the specimen was then naturally spread out and pinned to a flat board with the mucosal side facing upward.
The clinical tumor boundary was determined on the basis of at least two visual and tactile examinations by the surgeons, referring to the findings of preoperative endoscopy and barium meal study. Furthermore, indigo carmine was sometimes applied to clarify the boundary. When we could not definitively determine the boundary, we consulted the endoscopists or pathologists.
Gross findings were written on the individual’s chart and the specimen was finally photographed against a scale. The clinical tumor (cTumor) size and gross PM length were referred to on the chart and additionally measured using these images to confirm them.
Pathological examination and determination of pathological PM length
Each specimen was fixed in a 20% buffered formalin solution for 24–96 h. Serial longitudinal sections of the tumor were prepared and subsequent pathological evaluation was performed according to the JCGC [12]. After pathological evaluation, the tumor was mapped using the images of the fixed specimen. The location of the pathological proximal boundary and the pathological PM length were determined using this mapping in the pathological report for daily practice. The pathological proximal boundary included not only continuous mucosal or submucosal lesions, but also intermittent lesions, such as lymphatic or vascular invasions.
Definition of parameters
In our previous studies, we defined the length of discrepancy between the gross and pathological proximal boundary of the tumor as the ΔPM, which indicates how long the pathological tumor boundary extends beyond the gross boundary towards the proximal resection stump [8,9,10]. In this study, the ΔPM was defined similarly. Details of the ΔPM are shown in Supplementary Fig. 2. In a pathologically negative PM, the ΔPM could be calculated as follows: ΔPM = gross PM length − pathologically negative PM length. When a PM was pathologically positive in the initial transection but negative in the additional transection, tumor lesions existed not only in the initially resected specimen but also in the additional resected specimen. Thus, the ΔPM could be calculated as follows: ΔPM = gross PM length in the initial specimen + pathologically positive PM length in the additional specimen. In patients undergoing intraoperative frozen section analysis, 3 mm, which was the width of specimens excised from the proximal stump for analysis, was added to this calculation.
Postoperative treatment and follow-up
After surgery, postoperative chemotherapy was considered necessary based on the results of the ACTS-GC, CLASSIC, and START-II studies, which revealed the effect of adjuvant treatment [14,15,16]. In brief, patients with pathological stage II or III disease postoperatively underwent S-1 monotherapy for 1 year, capecitabine/S-1 plus oxaliplatin for 6 months, or docetaxel plus S-1 for 1 year. Blood tests, including carcinoembryonic antigen and carbohydrate antigen 19-9 levels, were undertaken for at least 3 months, and enhanced thoraco-abdominal computed tomography was performed every 3–6 months.
Analysis
We mainly performed three analyses for the enrolled patients. First, we determined the optimal cutoff level of ΔPM, applying receiver operating characteristic (ROC) analysis to the survival status, and categorized the patients into two groups: a longer (over the cutoff level) group and a shorter group (equal or under the cutoff level). We analyzed clinicopathological characteristics and compared them between the two groups. Second, we compared survival outcomes between the two groups. Furthermore, to identify pathological prognostic factors of overall survival (OS) and recurrence-free survival (RFS), we analyzed which factors were associated with poor survival by univariate and multivariate Cox regression analyses. In the univariate analysis, age (≥ 75 vs < 75 years), sex (male vs female), ΔPM (long vs short), pathological depth of tumor invasion (pT; ≥ T2 vs T1), pathological lymph node metastasis (pN; positive vs negative), pathological tumor size (≥ 80 mm vs < 80 mm), histopathological type (Und vs Dif), pathological PM (pPM; ≤ 28 mm vs > 28 mm), pathological EI (pEI; positive vs negative), growth type (Inf vs Sup or Exp), location of short axis (Circ vs others), and Borrmann classification (type 4 vs others) were inputted as variables. Regarding pathological PM, applying ROC analysis to the survival status determined 28 mm as the cutoff with area under the curve values of 0.578. In the multivariate analysis, variates that were generally recognized to be associated with survival outcomes or peritoneal metastasis were input as covariates. Furthermore, we compared recurrence patterns between the two groups. Third, we identified gross prognostic factors influencing OS in the long ΔPM group, because we assumed that we can intraoperatively obtain and use information of ΔPM by frozen section analysis. We analyzed which gross factors were associated with poor survival of the long ΔPM group using univariate and multivariate Cox regression analyses similar to the second analysis.
Statistical analysis
Data on the patients’ clinicopathological parameters, including tumor stage, were obtained by reviewing their medical charts. Survival curves were constructed using the Kaplan–Meier method and analyzed with the log-rank test. Differences between variables were analyzed using the χ2 test, Cox regression analysis, or logistic regression analysis, as appropriate. All analyses were performed with JMP® 13 (SAS Institute Inc., Cary, NC, USA), and p values of < 0.05 were considered statistically significant.
Results
Histogram and cutoff level of ΔPM
A total of 954 patients was finally enrolled in this study. A pathologically negative PM was observed in 911 patients in the initial transection of the esophagus, while a pathologically positive PM was observed in the remaining 43 patients, who underwent an additional transection to obtain a pathologically negative PM. A histogram of ΔPM in all the patients is shown in Fig. 1. Over 75% of the patients had ΔPM values between − 10 mm > and < 10 mm, and 8 mm was determined as the cutoff level of ΔPM with an area under the curve value of 0.555. The ΔPM > 8 mm and ≤ 8 mm groups were determined based on the cutoff level.
Comparison of clinicopathological features between the ΔPM > 8 mm and ≤ 8 mm groups
We compared clinical features between patients in the ΔPM > 8 mm and ≤ 8 mm groups, which contained 214 and 740 patients, respectively. The clinical and pathological characteristics of the groups are summarized in Table 1. More tumors in the ΔPM > 8 mm group were large, infiltrative, undifferentiated, deep, and associated with esophageal invasion than those in the ΔPM ≤ 8 mm group.
Survival outcomes of the ΔPM ≤ 8 mm and > 8 mm groups
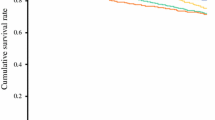
Figure 2 demonstrates that the OS of the ΔPM > 8 mm group was significantly worse than that of the ΔPM ≤ 8 mm group (5-year OS: 58% vs 78%; p < 0.0001). In the ΔPM > 8 mm group, 64 patients died of gastric cancer and 1 died of another disease, while in the ΔPM ≤ 8 mm group, 133 patients died of gastric cancer and 3 died of other diseases. Supplementary Fig. 3 also reveals that the RFS of the ΔPM > 8 mm group was significantly worse than that of the ΔPM ≤ 8 mm group (5-year RFS: 55% vs 76%; p < 0.0001).
Univariate and multivariate analyses and risk factors for OS and RFS
Univariate and multivariate analyses for OS and RFS are shown in Table 2 and Supplementary Table 1. In both OS and RFS, multivariate analysis identified that ΔPM > 8 mm was an independent prognostic factor. Furthermore, age ≥ 75 years, pT ≥ pT2, pN positivity, pTumor size > 80 mm, pEI positivity, Circ, and type 4 were also identified as independent prognostic factors (Table 2 and Supplementary Table 1).
Subgroup analysis of survival outcomes
Forest plots with hazard ratios (HRs) for OS according to pT status are shown in Supplementary Fig. 4. The HR of pT2–4 disease indicated that the OS of the ΔPM ≤ 8 mm group was better, while that of pT1 disease indicated that neither the OS of ΔPM > 8 mm and ≤ 8 mm groups was better. The likelihood ratio test revealed a significant interaction between pT status and ΔPM (p = 0.0007).
Patterns of recurrence according to ΔPM and risk factors
The patterns of first recurrence site according to ΔPM were analyzed (Supplementary Table 2). The incidence of peritoneal metastasis was the highest in both the ΔPM > 8 mm and ≤ 8 mm groups. Furthermore, the incidence of peritoneal metastasis in the ΔPM > 8 mm group was much higher than that in the ΔPM ≤ 8 mm group (p < 0.001).
Univariate and multivariate analyses revealed that ΔPM > 8 mm was an independent risk factor of peritoneal metastasis. pT4, pN positivity, Und, Inf, Circ, and type 4 were also identified as independent risk factors (Table 3).
Gross factors associated with poor survival in the ΔPM > 8 mm group
Univariate and multivariate analyses using gross predictors in the ΔPM > 8 mm group showed that cT4, cN positivity, gross EI (cEI) positivity, and Circ were independent risk factors for poor survival outcome (Table 4).
Survival outcomes according to the gross risk factors in the ΔPM > 8 mm group were analyzed. The 5-year OS rates were 35%, 42%, 11%, and 8% in patients with cT4, cN positivity, Circ, and cEI positivity, respectively (Fig. 3A, B, C, D).
Comparison of OS between cT4 and cT1–3 (A), cN-positive and -negative (B), Circ and others (C), and cEI-positive and -negative (D). Kaplan–Meier analyses showed that the OS of patients with cT4, cN positivity, Circ, or cEI positivity (red line) was significantly worse than those with cT1–3, cN negativity, others, or cEI negativity (blue line) (p < 0.0001). OS overall survival, Circ circumferential tumor, cEI gross esophageal invasion
Discussion
In this retrospective comparative study, we focused on the oncological impact of ΔPM in gastric cancer that requires total gastrectomy. Three novel findings were obtained from this study. First, the cutoff level of ΔPM based on survival was 8 mm. Tumor size, growth pattern, pathological type, depth, and esophageal invasion were associated with ΔPM > 8 mm. ΔPM > 8 mm was an independent risk factor for poorer survival outcomes, and a significant interaction was found between pT status and ΔPM. Second, ΔPM > 8 mm was associated with peritoneal metastasis but not local recurrence. Third, ΔPM > 8 mm combined with Circ or cEI positivity was associated with relatively poor survival outcome. These new findings imply that tumors with long ΔPMs have more malignant potential for peritoneal metastasis, thereby leading to poor survival. In addition to the depth of tumor invasion, growth type, size of tumor, short axis location, and pathological type, the ΔPM was a locally identified risk factor of survival outcome. This information may be useful intraoperatively when it is combined with several clinical factors. ΔPM is not only a tool to determine a gross PM length that provides pathological negativity in each type of gastric cancer [8,9,10], but also an indicator of the biological activity of the tumor.
The finding that patients whose tumors had unexpected horizontal spread have poor survival may be empirically known to many surgeons. However, the threshold of 8 mm is unexpectedly short. To the best of our knowledge, this is the first study to report what degree of ΔPM that means unexpected horizontal tumor spread is associated with survival outcome. However, similar results have been reported. In gastric cancer patients with positive resection margin, distant metastasis was the most common site of recurrence, while local recurrence was almost never found [17]. Although a positive margin often depends on technical issues, surgeons sometimes experience a positive margin despite adequate procedures. These experiences are only associated with a long ΔPM and suggest that aggressive tumor biology might be the real reason for positive margins and poor survival [18, 19]. The findings indicate that the horizontal spread of the primary tumor is a prognostic factor, similar to vertical invasion, and occult local spread involves occult systemic spread.
The findings identified in this study may particularly interest surgeons. However, ΔPM seems to be a fuzzy parameter. ΔPM consists of the gross PM length, which depends on determining where the proximal boundary is. Determining the proximal boundary is sometimes difficult and can change according to who evaluates it. Thus, the proximal boundary is essentially ambiguous, and ΔPM may lack objectivity. However, in this study, we determined nearly 1000 ΔPMs based on almost 1000 proximal boundaries. The proximal boundary in each surgery is always determined by at least two surgeons. Furthermore, different surgeons performed each surgery during the study period of 14 years. Therefore, although 8 mm, which we identified as a threshold of ΔPM in this study, may seem fuzzy and fragile, we believe that it is considerably homogenized and has reliable significance.
Several studies have examined the relationship between PM length and the survival outcome of patients with gastric cancer, but the results were inconsistent [20,21,22,23,24]. Some studies revealed pPM length was associated with survival outcome [20, 21]. Others revealed that they were not related [22,23,24]. In this study, pPM length was a prognostic factor in the univariate analysis. However, pPM length was not an independent prognostic factor. As the ΔPM becomes longer, the pPM length naturally becomes shorter. This study revealed that only ΔPM length was an independent prognostic factor. Therefore, aggressive tumor biology involving a long ΔPM might be an essential factor that may imply that a short pPM length is associated with poor survival. On the basis of the results of this study, pPM length does not influence survival outcome, although the issue remains controversial.
Whether the results will be used in daily practice may determine the value of this study. Even if it is postoperatively identified that patients have a ΔPM > 8 mm and are supposed to have poor survival, nothing other than careful surveillance can be undertaken at present. In the Japanese standard, the type of postoperative chemotherapy is determined solely according to the pathological stage. No other factors are considered in the selection of chemotherapy. Thus, there is no specific treatment for patients who have a ΔPM > 8 mm. However, if specific characteristics of tumors with occult horizontal spread are identified in the future, drugs corresponding to these characteristics can be used. Furthermore, information regarding the ΔPM may be used intraoperatively on a limited basis when a PM in the initial transection is pathologically positive by intraoperative frozen section analysis despite a gross PM length of > 8 mm, which indicates a ΔPM > 8 mm. In this study, disease with ΔPM > 8 mm and cEI positivity or Circ had a poorer survival outcome, at approximately 10% for 5-year OS. It presumably has an aggressive tumor biology, leading to a relatively poor survival outcome even if it is locally completely removed. This information may be a good reference as to whether an additional resection should be made when ΔPM > 8 mm is determined by frozen section analysis. However, the survival of patients with pT1 disease was not influenced by ΔPM. This finding indicates that additional resection to achieve an R0 resection can completely salvage patients who have pT1 disease if an intraoperative or postoperative pathological examination reveals incomplete tumor removal because of a long ΔPM. This is compatible with the results of our previous study [11] and our thoughts based on clinical experiences.
Our study has several limitations. First, it was a single institutional and retrospective study, although nearly 1000 patients were enrolled. However, it would be difficult to prospectively conduct this study, and the disadvantages of its retrospective nature may be minimal, because we retrospectively analyzed the data, most of which were prospectively collected. Second, we included only patients who underwent total gastrectomy. Patients who underwent other procedures such as distal gastrectomy were not included. The location of objected tumors and the dissection of lymph node stations differ in other types of gastrectomy, which would be subject to biases of survival. Therefore, whether the same is true in patients who underwent other types of gastrectomy remains unclear. Third, in the pathological examination, whole tumor sections were not always performed in daily practice. Thus, the longest pathological extension could exist at sites, where a section was not made for pathological evaluation, potentially resulting in overestimation of the pathological PM length. Finally, the therapeutic strategy for advanced gastric cancer is quite different between Western countries and Japan. Patients with advanced gastric cancer in Western countries usually undergo preoperative chemotherapy. However, most Japanese patients do not undergo such treatment and receive postoperative adjuvant chemotherapy. In this study, we excluded patients who underwent preoperative chemotherapy, because their gross and pathological tumor boundaries must be influenced by preoperative chemotherapy. Thus, the information identified in this study is not directly applicable to Western daily practice.
Despite these limitations, unexpected horizontal tumor spread of gastric cancer influences survival outcomes. Tumors with a long local extension may have high malignant potential involving distant metastasis, especially peritoneal metastasis, except for pT1 disease. For patients with pT2–4 disease and a long ΔPM, more careful surveillance or intensive treatment may be required postoperatively. Furthermore, intraoperatively, knowledge regarding the oncological characteristics of ΔPM and influencing factors of survival may be useful as a reference to indications of additional resection for pathologically positive PMs.
Availability of data and materials
The data sets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Bozzetti F, Bonfanti G, Bufalino R, Menotti V, Persano S, Andreola S, Doci R, Gennari L. Adequacy of margins of resection in gastrectomy for cancer. Ann Surg. 1982;196:682–90.
Ha TK, Kwon SJ. Clinical importance of the resection margin distance in gastric cancer patients. J Korean Gastric Cancer Assoc. 2006;6:277–83.
Bissolati M, Desio M, Rosa F, Rausei S, Chiari D, Molfino S, et al. Risk factor analysis for involvement of resection margins in gastric and esophagogastric junction cancer: an Italian multicenter study. Gastric Cancer. 2017;20:70–82.
Hallissey MT, Jewkes AJ, Dunn JA, Ward L, Fielding JW, et al. Resection-line involvement after gastric cancer: a continuing problem. Br J Surg. 1993;80:1418–20.
Bickenbach KA, Gonen M, Strong V, Brennan MF, Coit DG. Association of positive transection margins with gastric cancer survival and local recurrence. Ann Surg Oncol. 2013;20:2663–8.
Morgagni P, Garcea D, Marrelli D, De Manzoni G, Natalini G, Kurihara H, et al. Resection line involvement after gastric cancer surgery: clinical outcome in nonsurgically retreated patients. World J Surg. 2008;32:2661–7.
Hayami M, Ohashi M, Kumagai K, Sano T, Hiki N, Nunobe S, et al. A “Just Enough” gross proximal margin length ensuring pathologically complete resection in distal gastrectomy for gastric cancer. Ann Surg. 2020;1(2):pe026.
Koterazawa Y, Ohashi M, Hayami S, Kumagai K, Sano T, Nunobe S, et al. Minimum esophageal resection length to ensure negative proximal margin in total gastrectomy for gastric cancer. Ann Surg Open. 2022;3(1):pe127.
Koterazawa Y, Ohashi M, Hayami M, Makuuchi R, Ida S, Kumagai K, et al. Required esophageal resection length beyond the tumor boundary to ensure a negative proximal margin for gastric cancer with gross esophageal invasion or esophagogastric junction cancer. Gastric Cancer. 2023;26:451–9 (Epub ahead of print).
Muneoka Y, Ohashi M, Ishizuka N, Hayami M, Makuuchi R, Ida S, et al. Risk factors and oncological impact of positive resection margins in gastrectomy for cancer: are they salvaged by an additional resection? Gastric Cancer. 2022;25:287–96.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (ver. 5). Gastric Cancer. 2021;24:1–21.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, CLASSIC trial investigators, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21.
Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37:1296–304.
Wang SY, Yeh CN, Lee HL, Liu YY, Chao TC, Hwang TL, et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann Surg Oncol. 2009;16:2738–43.
Stiekema J, Cats A, Kuijpers A, Coevorden F, Boot H, Jansen EP, et al. Surgical treatment results of intestinal and diffuse type gastric cancer. Implications for a differentiated therapeutic approach? Eur J Surg Oncol. 2013;39:686–93.
Blackham AU, Swords DS, Levine EA, Fino NF, Squires MH, Poultsides G, et al. Is linitis plastic a contraindication for surgical resection: a multi-institution study of the U.S. Gastric Cancer Collaborative. Ann Surg Oncol. 2016;23:1203–11.
Squires MH 3rd, Kooby DA, Poultsides GA, Pawlik TM, Saunders N, Jin LX, et al. Is it time to abandon the 5-cm margin rule during resection of distal gastric adenocarcinoma? A multi-institution study of the U.S. Gastric Cancer Collaborative. Ann Surg Oncol. 2015;22:1243–51.
Hayami M, Ohashi M, Ishizuka N, Hiki N, Kumagai K, Souya N, et al. Oncological impact of gross proximal margin length in distal gastrectomy for gastric cancer: is the Japanese recommendation valid? Ann Surg Open. 2021;2(1):pe036.
Berlth F, Kim WH, Choi JH, Park SH, Kong SH, Lee HJ, et al. Prognostic impact of frozen section investigation and extent of proximal safety margin in gastric cancer resection. Ann Surg. 2020;272:871–8.
Ohe H, Lee WY, Hong SW, Chang YG, Lee B. Prognostic value of the distance of proximal resection margin in patients who have undergone curative gastric cancer surgery. World J Surg Oncol. 2014;23(12):296.
Kim MG, Lee JH, Ha TK, Kwon SJ. The distance of proximal resection margin dose not significantly influence on the prognosis of gastric cancer patients after curative resection. Ann Surg Treat Res. 2014;87:223–31.
Acknowledgements
We thank Manabu Takamatsu from the Department of Pathology, Cancer Institute Hospital, for reviewing a draft of the descriptions regarding pathological issues in this manuscript.
Author information
Authors and Affiliations
Contributions
YK and MO: designed the protocol and drafted the manuscript, and all other authors participated in the design of the study. YK and MO: analyzed the data. MH, RM, SI, KK, TS, and SN: interpreted the data and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10120_2023_1401_MOESM3_ESM.tif
Supplementary file3 Fig. 1 Patient flowchart of the enrollment process. From January 2005 to December 2018, 1493 patients who had gastric cancer, gastric cancer with esophageal invasion, or Siewert type III esophagogastric junction cancer underwent total gastrectomy or proximal gastrectomy. After some patients were excluded, 954 patients were finally enrolled in this study. (TIF 87 KB)
10120_2023_1401_MOESM4_ESM.tif
Supplementary file4 Fig. 2 Definition of parameters used in this study. We defined several parameters as per our previous study. ΔPM indicates the length of discrepancies between clinical and pathological PM lengths in cases of (A) pathologically negative PM and (B) pathologically positive PM. PM, proximal margin. (TIF 110 KB)
10120_2023_1401_MOESM5_ESM.tif
Supplementary file5 Fig. 3 Comparison of RFS between the ΔPM >8 mm and ≤8 mm groups. Kaplan–Meier analyses showed that the RFS of the ΔPM >8 mm group (red line) was significantly worse (p < 0.0001) than that of the ΔPM ≤8 mm group (blue line). RFS, recurrence-free survival. (TIF 105 KB)
10120_2023_1401_MOESM6_ESM.tif
Supplementary file6 Fig. 4 Forest plot showing HRs of OS according to pT status. The HR of pT2–4 disease indicated that the OS of the ΔPM ≤8 mm group was better, and that of pT1 disease indicated that neither the OS of the ΔPM >8 mm and ≤8 mm groups was better. HR, hazard ratio; OS, overall survival. (TIF 64 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koterazawa, Y., Ohashi, M., Hayami, M. et al. Oncological impact of unexpected horizontal tumor spread in gastric cancer that requires total gastrectomy. Gastric Cancer 26, 823–832 (2023). https://doi.org/10.1007/s10120-023-01401-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01401-5