Abstract
Background
Although a 3–5 cm surgical margin distance is recommended for advanced gastric cancer (GC) in Japanese guidelines, little is known about the clinical effects of the surgical margin, especially the distal resection margin (DM). This study aims to clarify the clinical significance of DM in GC.
Methods
A total of 415 GC patients who underwent curative distal gastrectomy between 2008 and 2018 were analyzed retrospectively.
Results
The DM significantly stratified recurrence-free survival (P = 0.002), and a DM < 30 mm was an independent factor of a poor prognosis (P = 0.023, hazard ratio: 1.91). Lymphatic recurrence occurred significantly more frequently in the DM < 30 mm group than in the DM ≥ 30 mm group (P = 0.019, 6.9% vs. 1.9%). Regarding the station No.6 lymph node metastases in advanced GC (DM < 30 mm vs. 30 mm ≤ DM ≤ 50 mm vs. DM > 50 mm), the number (P < 0.001, 1.42 ± 1.69 vs. 1.18 ± 1.80 vs. 0.18 ± 0.64), the positive rate (P < 0.001, 59.0% vs. 46.7% vs. 11.3%) and therapeutic value index (43.3 vs. 14.5 vs. 8.0) were significantly higher in the DM < 30 mm group. By subdivision using the DM distance of 30 mm, more segmented prognostic stratifications were possible (P < 0.001).
Conclusions
A DM of less than 30 mm could be a surrogate marker of poor RFS, especially increasing nodal recurrence. More intensive treatment strategies, including lymphadenectomy and chemotherapy, are needed for patients with this condition.
Similar content being viewed by others
Background
Gastric cancer (GC) is one of the most common cancer types and a leading cause of death worldwide [1]. Although various treatments for GC have improved considerably in recent decades [2,3,4], curative gastrectomy with regional lymph node (LN) dissection remains the primary treatment for patients with resectable GC [5,6,7]. The surgical procedure recommends a surgical margin of sufficient distance to achieve no residual cancer of the resection line because microscopic positive resection margins have been associated with a poor prognosis for GC [8,9,10,11]. However, in patients with a negative surgical margin, it is unclear whether the distance, especially the distal resection margin (DM), affects cancer progression and prognosis after distal GC. A sufficient surgical margin may indicate a more sufficient lymphadenectomy and contribute to adequate local tumor clearance and curability of surrounding metastatic LNs. Indeed, Mine et al. suggested that a proximal margin of more than 2 cm affects a favorable prognosis in patients with Siewert type II and III adenocarcinomas of the esophagogastric junction [12].
Because cancer cells spread stepwise from the peri-gastric area [13], a longer surgical margin may indicate sufficient local tumor clearance to prevent LN metastases (LNM) and perinodal involvement of cancer cells[14]. Regarding the proximal margin, various previous studies have indicated the prognostic effect of a sufficient proximal margin in GC [15,16,17,18]. However, there have been no reports of the surgical curability of surrounding LNs and the prognostic effect of DM distance. In the present study, we investigated these issues. Our results suggested that DM distance might be a surrogate marker of local cancer progression and prognosis and could be an indicator of intensive treatments for GC patients.
Methods
Patients
This study was approved by the Kyoto Prefectural University of Medicine and was performed under the ethical standards described in the Declaration of Helsinki. Informed written consent was obtained from all patients. A total of 559 consecutive patients underwent R0 curative distal gastrectomy with lymphadenectomy for GC at our institute between January 2008 and June 2018. Of these, 144 were excluded from this study due to having multiple carcinomas (n = 60), post-endoscopic resection (n = 82), or neoadjuvant chemotherapy (n = 2). Therefore, we investigated 415 consecutive patients (Fig. 1). The follow-up program after gastrectomy consisted of regular physical examinations and laboratory tests, chest X-rays, an upper gastrointestinal series or endoscopy, and ultrasonography or computer tomography for the first 5 years and yearly endoscopies thereafter, if possible. The clinicopathological findings of these patients were retrospectively obtained based on their medical records.
Definition of the surgical margin
Resected specimens were examined by pathologists and evaluated based on the 15th Japanese Classification of Gastric Carcinoma (JCGC) [19]. All dissected specimens, including the stomach and LNs, were fixed in buffered formalin, embedded in paraffin, and subjected to pathological examination. Pathologists in our institution examined embedded LNs by sectioning slices in the plane of the largest node dimension to confirm the presence of metastasis. The DM distance was defined as the shortest distance from the most distal tumor end to the distal resection line, measured on formalin-fixed surgical specimens. We defined pT2 or deeper, and/or pN1 or higher as advanced GC, and the remaining pT1 cancers as early GC.
Statistical analysis
Statistical analyses were conducted using JMP version 16 (SAS Institute Inc., Cary, NC, USA). Mann–Whitney U tests for unpaired continuous data were used to compare clinicopathological variables. For survival analysis, Kaplan–Meier survival curves were constructed for groups based on univariate predictors, and differences between the groups were tested using generalized Wilcoxon tests. A Cox proportional hazards model was used to further evaluate the multivariate survival analysis. Statistical significance was set at P < 0.05.
Results
Shorter DM distance showed poor prognosis in patients who received curative distal gastrectomy
Firstly, we investigated differences in the prognosis of patients who received curative distal gastrectomy according to the DM distance using cut-off values of 30 mm and 50 mm, which are recommended as surgical margins for localized GC (Type 1 or 2) and diffuse GC (Type 3 or 4) in the Japanese guidelines. As a result, the DM distance significantly stratified recurrence-free survival (RFS), and the DM distance < 30 mm group showed the worst RFS rate (DM distance < 30 mm vs. 30 mm ≤ DM distance ≤ 50 mm vs. DM distance > 50 mm: 77.0% vs. 84.2% vs. 92.8%, P = 0.002; Figure S1). Next, we investigated the prognosis of GC patients separately according to the degree of cancer progression using a cut-off value of 30 mm (Fig. 2). In advanced GC, patients with a DM distance < 30 mm had significantly poorer RFS than those with a DM distance ≥ 30 mm (DM distance < 30 mm vs. DM distance ≥ 30 mm: 63.7% vs. 80.6%, P = 0.040), while there was no significant difference between both groups in early GC (RFS: DM distance < 30 mm vs. DM distance ≥ 30 mm: 100% vs. 96.2%, P = 0.238).
Comparisons of survival curves according to the distal resection margin (DM) distance. A Comparisons of overall survival (OS) and recurrence-free survival (RFS) in gastric cancer patients who underwent distal gastrectomy. B Comparisons of OS and RFS in early gastric cancer patients who underwent distal gastrectomy. C Comparisons of OS and RFS in advanced gastric cancer patients who underwent distal gastrectomy
Univariate and multivariate analyses of DM distance using a Cox proportional hazard model
To elucidate the prognostic factors of RFS in patients who underwent distal gastrectomy, we performed univariate and multivariate analyses using a Cox proportional hazard model. As shown in Table 1, the clinical variables included pStage, histological type, venous invasion, lymphatic invasion, adjuvant chemotherapy, tumor axis, and distal surgical margin distance. The median tumor axis in this cohort was 35 mm (Interquartile range, 23–53), therefore, we used 35 mm as the cut-off. Consequently, univariate and multivariate analyses showed that a DM distance < 30 mm was an independent poor prognostic factor for RFS (P = 0.023, hazard ratio [HR]: 1.91, 95% confidence interval [CI]: 1.09–3.33) as well as a factor of advanced pStage.
Comparisons of clinicopathological factors between the DM distance < 30 mm group and the DM distance ≥ 30 mm group
Next, we compared the clinicopathological characteristics of the DM distance < 30 mm group and the DM distance ≥ 30 mm group (Table 2). There was no difference between the groups in patients with advanced GC, except for the proportion of tumors located in the upper stomach (P < 0.001) and larger tumor axis (P = 0.005). A DM distance < 30 mm was significantly correlated with the differentiated type (P = 0.023) and a higher rate of positive lymphatic invasion (P = 0.036) compared to patients with early GC with a DM distance ≥ 30 mm. Besides, multivariate analysis using the Cox proportional hazard model to assess the prognostic factors of RFS in advanced GC showed that a DM distance < 30 mm, rather than a larger tumor axis, was identified as an independent poor prognostic factor (P = 0.019, hazard ratio [HR]: 2.10, 95% confidence interval [CI]: 1.13–3.88; Table S1).
Contribution of a shorter DM distance to station No.6 LNM
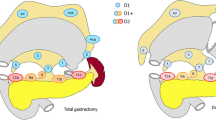
Regarding the patterns of recurrence, the incidence of lymphatic recurrence was significantly higher in patients with a DM distance < 30 mm compared to those with a DM distance ≥ 30 mm (DM distance < 30 mm vs. DM distance ≥ 30 mm: 6.9% vs. 1.9%, P = 0.019; Table 3). Among seven cases of lymphatic recurrence in the DM distance < 30 mm group, six cases occurred in the para-aortic LN station and the hepatoduodenal ligament LN station. To investigate the mechanism by which a shorter DM distance is associated with a higher frequency of LN recurrence, we compared the frequency of the LNMs in each station. In the patients with advanced GC, the LNMs in station No.6 occurred significantly more frequently (DM distance < 30 mm vs. 30 mm ≤ DM distance ≤ 50 mm vs. DM distance > 50 mm: 59.0% vs. 46.7% vs. 11.3%, P < 0.001) and the number of LNMs in station No.6 was significantly higher (DM distance < 30 mm vs. 30 mm ≤ DM distance ≤ 50 mm vs. DM distance > 50 mm: 1.42 ± 1.69 vs. 1.18 ± 1.80 vs. 0.18 ± 0.64, P < 0.001) in the DM distance < 30 mm group than in the DM distance ≥ 30 mm group (Fig. 3). To determine more accurately whether the ease of metastasis to the No.6 lymph node is dependent on the DM distance, we performed subgroup analysis among the patients with pN1. This analysis showed that LNM in station No.6 occurred more frequently in the DM distance < 30 mm group than in the other groups (DM distance < 30 mm vs. 30 mm ≤ DM distance ≤ 50 mm vs. DM distance > 50 mm: 75.0% vs. 45.5% vs. 13.3%, P = 0.003; Table S2). In addition, the therapeutic value index was estimated by multiplying the metastasis rate by the 5-year overall survival rate in patients with metastasis in the respective nodes [20]. As a result, the therapeutic value index in station No.6 LNs was highest in the DM distance < 30 mm group than in the other two groups in advanced GC (DM distance < 30 mm vs. 30 mm ≤ DM distance ≤ 50 mm vs. DM distance > 50 mm: 43.3 vs. 14.5 vs. 8.0; Table 4).
Comparisons of the incidence of lymph node metastasis between advanced gastric cancer (GC) patients with a distal resection margin (DM) distance ≥ 30 mm and a DM distance < 30 mm. A In advanced GC, there was a higher incidence of lymph node metastasis at station No.6 in the DM distance < 30 mm group compared to the DM distance ≥ 30 mm. B The number of lymph node metastases at station No.6 was higher in the DM distance < 30 mm group compared to the DM distance ≥ 30 mm and the DM distance ≤ 50 mm group, and the DM > 50 mm group
Evaluation of the more precise staging system using a 30 mm cut-off value for the DM distance
Next, we examined survival curves for combinations of the staging system in JCGC with a DM distance of 30 mm. More segmented prognostic stratifications were observed (RFS; pStage II, DM distance < 30 mm vs. pStage II, DM distance ≥ 30 mm vs. pStage III, DM distance < 30 mm vs. pStage III, DM distance ≥ 30 mm: 86.4% vs. 73.0% vs. 57.1% vs. 35.3%, P < 0.001; Fig. 4). This result suggested that a cut-off value for the DM distance of < 30 mm could be a useful indicator for stratifying prognosis as well as the traditional staging system in patients with pStage II or III GC.
Recurrence-free survival curves with combinations of the staging system of the Japanese classification of gastric carcinoma (JCGC) and the distal resection margin (DM) distance. Greater segmented prognostic stratification was possible with combinations of the staging system in JCGC and using a DM distance cut-off value of 30 mm (RFS; pStage IB, DM < 30 mm vs. pStage IB, DM ≥ 30 mm, vs. pStage II, DM < 30 mm vs. pStage II, DM ≥ 30 mm vs. pStage III, DM < 30 mm vs. pStage III, DM ≥ 30 mm = 94.1% vs. 93.2% vs. 73.0% vs. 86.4% vs. 35.3% vs. 57.1%, P < 0.001)
Discussion
The Japanese Gastric Cancer Association (JGCA) recommends a margin of 2 cm for T1 tumors, 3 cm for T2 or deeper tumors with an expansive growth pattern, and 5 cm for T2 or deeper tumors with an infiltrative growth pattern [6]. On the other hand, the European Society for Medical Oncology indicates an appropriate proximal surgical margin of 5–8 cm [5], and The National Comprehensive Cancer Network guidelines suggest an adequate surgical margin of 4 cm for T1b-T3 tumors to achieve negative microscopic margin [21]. Although some clinical studies have investigated the prognostic significance of proximal surgical margin distance [17, 18, 22,23,24,25,26], to our knowledge, there have been no reports of the prognostic and treatment significance of the DM distance. In this study, we first investigate the prognostic effect of the DM distance using the cut-off of 3 cm and 5 cm as cut-off values based on Japanese guidelines and previous studies on proximal margin distances [18, 25] and found that the patients with a DM distance of less than 3 cm had a significantly worse prognosis. Therefore, we selected 3 cm as the cut-off value for further analyses. Then, we clearly demonstrated that a DM distance < 30 mm was an independent factor of a poor prognosis (HR: 1.91, 95% CI: 1.09–3.33) and an indicator of excessive nodal metastases around the No.6 LN station. These findings strongly suggested that DM distance could be an important surrogate marker for nodal metastasis and poor prognosis, applying more intensive lymphadenectomy and adjuvant therapy for advanced GC patients.
Previously, Maspero et al. retrospectively investigated the adequacy of the resection margin in 279 stage I – III GC patients and proved that adequate margins contributed to favorable OS, RFS, and local recurrence rates [26]. Another two reports also demonstrated that a longer proximal surgical margin distance contributed to long-term prognosis for GC [17, 24]. However, Kim et al. demonstrated that proximal margin distance did not affect OS and RFS in multivariate analysis using a Cox proportion hazard model in a large cohort of 859 cases of distal gastrectomy and 659 cases of total gastrectomy, and another two studies also stated similar results [22, 23]. Although one study found that the proximal margin distance contributed to curability [15], most studies do not support this outcome. Therefore, the curative and prognostic effects of proximal margin distance are still controversial. Regarding the distal resection margin, little is known about its clinical effects. In this study, we clearly demonstrated that a shorter DM distance contributed to lymphatic spreading as well as a poorer prognosis.
The most striking finding in the present study was that lymphatic recurrence occurred more frequently in patients with a DM distance < 30 mm compared to a DM ≥ 30 mm, and a positive rate of LNMs at station No.6 (DM distance < 30 mm vs. 30 mm ≤ DM distance ≤ 50 mm vs. DM distance > 50 mm: 59.0% vs. 46.7% vs. 11.3%, P < 0.001) in advanced GC, which might indicate a putative additional lymphatic flow to station No.14v and No.16 LNs outside the LN resection area [6, 13], and the number of LNMs at station No.6 (P < 0.001: DM distance < 30 mm vs. 30 mm ≤ DM distance ≤ 50 mm vs. DM distance > 50 mm: 1.42 ± 1.69 vs. 1.18 ± 1.80 vs. 0.18 ± 0.64) was significantly higher in the DM distance < 30 mm group than in the DM distance ≥ 30 mm group. Thus, DM distance could be considered an indicator of the extent of lymphatic spreading in GC. In distal gastrectomy, unlike the proximal resection margin, the distal resection margin is subject to anatomical constraints that allow for relatively little adjustment. As a result, the distance of the distal margin can be considered an indicator of the degree of distal localization of the tumor. Indeed, in adenocarcinoma of the esophagogastric junction (EGJ), previous studies have investigated the relationship between mediastinal LNMs and the extent of esophageal invasion in adenocarcinoma and found that a longer distance from the EGJ to the proximal edge of the primary tumor contribute to middle or upper mediastinal LNs [27,28,29]. These findings strongly suggested the significance of the DM distance on the extent and pattern of nodal metastasis.
Regarding the therapeutic value of lymphadenectomy associated with the DM distance, the therapeutic value index of LNs at station No.6 was extremely higher in GC patients with a DM distance < 30 mm than in those with a DM distance ≥ 30 mm. This finding also indicated that GC patients with a DM distance < 30 mm may need additional lymphadenectomy around LNs at station No.6. Regarding D2 + lymphadenectomy, recent studies have clarified the significance of station No.13, 14v, and 16 LN dissection [30,31,32,33] in some advanced types of GC. Moreover, some studies have identified that LNM at station No.6 was an independent risk factor for station No.13 and 14v LNM [30, 31]. In our study, we demonstrated that the DM distance was a surrogate marker of the LNM pattern and served as an indicator to reconsider the extent of LN dissection.
It is crucial to note that our proposal is not advocating for the enlargement of the surgical resection margin; rather, it suggests a reevaluation of more suitable strategies, considering tumor laterality using such as the indicator of DM distance. In distal gastrectomy, anatomical constraints may make extending the surgical resection margin challenging in some instances. Consequently, in cases of distal gastric cancer where obtaining a sufficient DM distance is not feasible, it becomes necessary to consider broader lymph node dissection beyond the standard scope and/or more potent adjuvant chemotherapy methods, such as Cape-OX therapy, SOX therapy, and S-1 plus docetaxel therapy [34,35,36].
This study had some limitations. Firstly, because the results were obtained from a retrospective evaluation of a small number of patients at a single institute, the findings of the present study should be validated in a larger prospective multicenter study. Secondly, the DM distance was used in this study as an indicator of distal orientation; however, the actual duodenal resection length varies and needs to be validated in more detail. Thirdly, there was the potential variability in the measurement of margin distances due to different formalin immersion times, which can cause changes in tissue shrinkage. Fourthly, there was a discrepancy between RFS and OS among patients with advanced GC. The reason for this could be that of the 34 patients who experienced recurrence events, 4 (11.8%) have survived for more than 5 years due to the improvement and efficacy of recent chemotherapies for recurrences. It is also important to note that further research is needed, particularly with larger sample sizes and longer follow-up periods, to corroborate these findings. Nevertheless, our findings suggest the impact of DM distance on cancer progression and prognosis for GC patients due to local recurrence and lymphatic progression. Moreover, we should discuss that patients with a shorter DM distance could be sufficiently treated and obtain benefits from more extended lymphadenectomy and/or more intensive adjuvant therapy due to the higher incidence of LN involvement.
Conclusion
A shorter DM distance could be an indicator of poor prognosis in patients with advanced GC who underwent distal gastrectomy, increasing the frequency of metastasis to LNs at station No.6. Further studies are warranted to clarify the significance of additional extended lymphadenectomy around station No.6 LNs, such as station No.14v, and intensive adjuvant chemotherapy to improve the prognosis of patients with a shorter DM distance.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GC:
-
Gastric cancer
- RFS:
-
Recurrence-free survival
- DM:
-
Distal margin
- LN:
-
Lymph node
- JCGC:
-
Japanese Classification of Gastric Carcinoma
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Chen ZD, Zhang PF, Xi HQ, Wei B, Chen L, Tang Y. Recent advances in the diagnosis, staging, treatment, and prognosis of advanced gastric cancer: a literature review. Front Med (Lausanne). 2021;8:744839.
Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. 2020;23(4):565–78.
Harada K, Mizrak Kaya D, Shimodaira Y, Ajani JA. Global chemotherapy development for gastric cancer. Gastric Cancer. 2017;20(Suppl 1):92–101.
Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38–49.
Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1–21.
Komatsu S, Otsuji E. Essential updates 2017/2018: recent topics in the treatment and research of gastric cancer in Japan. Ann Gastroenterol Surg. 2019;3(6):581–91.
Cho BC, Jeung HC, Choi HJ, Rha SY, Hyung WJ, Cheong JH, Noh SH, Chung HC. Prognostic impact of resection margin involvement after extended (D2/D3) gastrectomy for advanced gastric cancer: a 15-year experience at a single institute. J Surg Oncol. 2007;95(6):461–8.
Sun Z, Li DM, Wang ZN, Huang BJ, Xu Y, Li K, Xu HM. Prognostic significance of microscopic positive margins for gastric cancer patients with potentially curative resection. Ann Surg Oncol. 2009;16(11):3028–37.
Nagata T, Ichikawa D, Komatsu S, Inoue K, Shiozaki A, Fujiwara H, Okamoto K, Sakakura C, Otsuji E. Prognostic impact of microscopic positive margin in gastric cancer patients. J Surg Oncol. 2011;104(6):592–7.
Zhao B, Lu H, Bao S, Luo R, Mei D, Xu H, Huang B. Impact of proximal resection margin involvement on survival outcome in patients with proximal gastric cancer. J Clin Pathol. 2020;73(8):470–5.
Mine S, Sano T, Hiki N, Yamada K, Kosuga T, Nunobe S, Yamaguchi T. Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg. 2013;100(8):1050–4.
Shida A, Mitsumori N, Fujioka S, Takano Y, Fujisaki M, Hashizume R, Takahashi N, Ishibashi Y, Yanaga K. Sentinel node navigation surgery for early gastric cancer: analysis of factors which affect direction of lymphatic drainage. World J Surg. 2018;42(3):766–72.
Koike H, Ichikawa D, Kitamura K, Tsuchihashi Y, Yamagishi H. Perinodal involvement of cancer cells in gastric cancer patients. Surgery. 2004;135(3):266–72.
Bozzetti F, Bonfanti G, Bufalino R, Menotti V, Persano S, Andreola S, Doci R, Gennari L. Adequacy of margins of resection in gastrectomy for cancer. Ann Surg. 1982;196(6):685–90.
Ito H, Clancy TE, Osteen RT, Swanson RS, Bueno R, Sugarbaker DJ, Ashley SW, Zinner MJ, Whang EE. Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg. 2004;199(6):880–6.
Luo J, Jiang Y, Chen X, Chen Y, Gurung JL, Mou T, Zhao L, Lyu G, Li T, Li G, et al. Prognostic value and nomograms of proximal margin distance in gastric cancer with radical distal gastrectomy. Chin J Cancer Res. 2020;32(2):186–96.
Kim A, Kim BS, Yook JH, Kim BS. Optimal proximal resection margin distance for gastrectomy in advanced gastric cancer. World J Gastroenterol. 2020;26(18):2232–46.
Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20(2):217–25.
Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82(3):346–51.
Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11(5):531–46.
Kim MG, Lee JH, Ha TK, Kwon SJ. The distance of proximal resection margin dose not significantly influence on the prognosis of gastric cancer patients after curative resection. Ann Surg Treat Res. 2014;87(5):223–31.
Gamboa-Hoil SI, Adrián PO, Rodrigo SM, Juan Carlos SG, Felix QC. Surgical margins in gastric cancer T2 and T3 and its relationship with recurrence and overall survival at 5 years. Surg Oncol. 2020;34:13–6.
Wang J, Liu J, Zhang G, Kong D. Individualized proximal margin correlates with outcomes in gastric cancers with radical gastrectomy. Tumour Biol. 2017;39(6):1010428317711032.
Squires MH 3rd, Kooby DA, Poultsides GA, Pawlik TM, Weber SM, Schmidt CR, Votanopoulos KI, Fields RC, Ejaz A, Acher AW, et al. Is it time to abandon the 5-cm margin rule during resection of distal gastric adenocarcinoma? A multi-institution study of the U.S Gastric Cancer Collaborative. Ann Surg Oncol. 2015;22(4):1243–51.
Maspero M, Sposito C, Benedetti A, Virdis M, Di Bartolomeo M, Milione M, Mazzaferro V. Impact of surgical margins on overall survival after gastrectomy for gastric cancer: a validation of Japanese Gastric Cancer Association guidelines on a Western series. Ann Surg Oncol. 2022;29(5):3096–108.
Kakeji Y, Yamamoto M, Ito S, Sugiyama M, Egashira A, Saeki H, Morita M, Sakaguchi Y, Toh Y, Maehara Y. Lymph node metastasis from cancer of the esophagogastric junction, and determination of the appropriate nodal dissection. Surg Today. 2012;42(4):351–8.
Kurokawa Y, Hiki N, Yoshikawa T, Kishi K, Ito Y, Ohi M, Wada N, Takiguchi S, Mine S, Hasegawa S, et al. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery. 2015;157(3):551–5.
Koyanagi K, Kato F, Kanamori J, Daiko H, Ozawa S, Tachimori Y. Clinical significance of esophageal invasion length for the prediction of mediastinal lymph node metastasis in Siewert type II adenocarcinoma: a retrospective single-institution study. Ann Gastroenterol Surg. 2018;2(3):187–96.
Masuda TA, Sakaguchi Y, Toh Y, Aoki Y, Harimoto N, Taomoto J, Ikeda O, Ohga T, Adachi E, Okamura T. Clinical characteristics of gastric cancer with metastasis to the lymph node along the superior mesenteric vein (14v). Dig Surg. 2008;25(5):351–8.
Wu L, Zhang C, Liang Y, Wang X, Ding X, Liang H. Risk factors for metastasis to No.14v lymph node and prognostic value of 14v status for gastric cancer patients after surgery. Jpn J Clin Oncol. 2018;48(4):335–42.
Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy–Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22(14):2767–73.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453–62.
Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol. 2021;32(3):368–74.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage iii gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304.
Acknowledgements
Not applicable.
Funding
There is no funding to be declared.
Author information
Authors and Affiliations
Contributions
Y.T. and S.K. designed this work. S.K., K.N., T.O., T.Ko., H.K., A.S., T.Ku., H.F., E.O. acquired clinical data and critically reviewed this manuscript. Y.T. and S.K. analyzed data and wrote the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was designed by the Declaration of Helsinki and was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine. All patients received a sufficient explanation of the study, and written informed consent was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Comparisons of recurrence-free survival curves according to the distal resection margin distance.
Additional file 2: Table S1.
Results of univariate and multivariate analyses using a Cox proportional hazard model in advanced gastric cancer. Table S2. Frequency of No.6 lymph node metastasis in patients with pN1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Takashima, Y., Komatsu, S., Nishibeppu, K. et al. A shorter distal resection margin is a surrogate marker of nodal metastasis and poor prognosis in distal gastrectomy for advanced gastric cancer. BMC Cancer 23, 1075 (2023). https://doi.org/10.1186/s12885-023-11570-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11570-2