Abstract
Background
Palliative radiotherapy seems to be rarely performed for incurable gastric cancer. In this first multicenter study, we examined the effectiveness of palliative radiotherapy and investigated whether biologically effective dose (BED) is associated with survival, response, or re-bleeding.
Methods
Eligibility criteria included blood transfusion or hemoglobin levels < 8.0 g/dL. The primary endpoint was the intention-to-treat (ITT) bleeding response rate at 4 weeks. Response entailed all of the following criteria: (i) hemoglobin levels ≥ 8.0 g/dL; (ii) 7 consecutive days without blood transfusion anytime between enrollment and blood sampling; and (iii) no salvage treatment (surgery, endoscopic treatment, transcatheter embolization, or re-irradiation) for bleeding gastric cancer. Re-bleeding was defined as the need for blood transfusion or salvage treatment.
Results
We enrolled 55 patients from 15 institutions. The ITT response rates were 47%, 53%, and 49% at 2, 4, and 8 weeks, respectively. The per-protocol response rates were 56%, 78%, and 90% at 2, 4, and 8 weeks, respectively. Neither response nor BED (α/β = 10) predicted overall survival. Multivariable Fine-Gray model showed that BED was not a significant predictor of response. Univariable Cox model showed that BED was not significantly associated with re-bleeding. Grades 1, 2, 3, and, ≥ 4 radiation-related adverse events were reported in 11, 9, 1, and 0 patients, respectively.
Conclusions
The per-protocol response rate increased to 90% during the 8-week follow-up. The frequent occurrence of death starting shortly after enrollment lowered the ITT response rate. BED was not associated with survival, bleeding response, or re-bleeding.
Similar content being viewed by others
Introduction
Gastric cancer is the fifth most common cancer and fourth most common cause of cancer-related death worldwide [1]. In Asian countries, many of which have screening programs in place, lesser proportions of patients with gastric cancer are diagnosed at an advanced stage than in Western countries [2]. Nonetheless, because gastric cancer is more prevalent in Asia, a substantial number of patients with gastric cancer present with advanced disease in Asian countries. Despite improvements in systemic therapies, patients with advanced gastric cancer still have limited survival. [2]. More than half of patients with non-resectable gastric cancer may experience tumor-related complications, which require intervention [3]. Bleeding from gastric cancer is a burdensome complication, which may require blood transfusion and hospitalization [4]. Palliative surgery is an effective treatment for bleeding, but is often difficult for elderly patients and those with a poor performance status [5]. Bulky primary tumors and/or metastatic lymph nodes that involve other organs may also sometimes hamper the palliative surgery. Endoscopic therapy and transcatheter arterial embolization are other options of treatment [6]. Palliative radiotherapy for bleeding gastric cancer has been studied for its effectiveness [7] but seems to be rarely performed in daily practice [8]. A Japanese Radiation Oncology Study Group (JROSG) survey found that in most of the 43 facilities investigated (median annual number of patients treated with radiotherapy, 594), only 0–1 patient per center was treated in a year with palliative radiotherapy for bleeding upper gastrointestinal tract cancer [8]. This underutilization of radiotherapy may be due to the paucity of high quality data on its effectiveness and safety. Past studies on palliative radiotherapy for gastric cancer are dominated by retrospective studies [7], although a few prospective studies investigated its effectiveness [9, 10]. Past studies on palliative radiotherapy for gastric cancer are also dominated by single-center studies, and to the best of our knowledge, this study is the first multicenter study to examine the effectiveness of palliative radiotherapy for gastric cancer. In this largest prospective study to date, we performed a longitudinal assessment of bleeding response and re-bleeding after palliative radiotherapy for gastric cancer. We additionally investigated whether higher biologically effective dose (BED) is associated with patient benefits in terms of survival, bleeding response, or re-bleeding. BED is calculated based on the fraction size and number [11] and can be used to compare the effect of different dose fractionations even when the fraction sizes are different (e.g., 8 Gy in 1 fraction vs. 30 Gy in 10 fractions).
Methods
Study design and patients
JROSG 17-3 was a multicenter prospective observational study. The study was registered with University hospital Medical Information Network (UMIN) Clinical Trial Registry, number UMIN000029580. Eligibility criteria included patients aged ≥ 20 years; pathologically proven gastric cancer; bleeding from primary or recurrent gastric cancer that was confirmed either endoscopically, by hematemesis, or by melena; patients who received blood transfusion or those with hemoglobin levels < 8.0 g/dL within 4 weeks before enrollment; and Eastern Cooperative Oncology Group performance status score of 0–3. Patients were excluded if the tumor scheduled to receive radiotherapy had been previously irradiated; the patient had received chemotherapy or molecular targeted therapy within 2 weeks before the planned initiation date of radiotherapy; or if the patient was scheduled to receive chemotherapy or molecular targeted therapy within 2 weeks after the planned initiation date of radiotherapy. All inclusion/exclusion criteria are listed in Supplementary Material. Dose prescription and target volume definition were determined at the discretion of the treating radiation oncologist. Systemic therapy, blood transfusion, and local salvage treatment for bleeding gastric cancer (surgery, endoscopic treatment, transcatheter embolization, and re-irradiation) concurrent with or after the radiotherapy for bleeding gastric cancer were allowed if deemed necessary by the treating physician. The protocol was approved by the participating centers' institutional review boards, and written informed consent was obtained from all participants.
Evaluation
We enrolled patients with anemia ≥ grade 3 according to the Common Terminology Criteria for Adverse Events (CTCAE) v 4.0 (i.e., hemoglobin level < 8.0 g/dL or requirement of blood transfusion). Bleeding response was defined as the occurrence of anemia ≤ grade 2 after radiotherapy. Specifically, patients were said to exhibit a response if (i) hemoglobin level was ≥ 8.0 g/dL, (ii) there were 7 consecutive days without blood transfusion anytime between enrollment and blood sampling, and (iii) they did not require local salvage treatment for bleeding gastric cancer (surgery, endoscopic treatment, transcatheter embolization, or re-irradiation) between enrollment and blood sampling. The primary endpoint of the study was the intention-to-treat (ITT) bleeding response rate (i.e., the denominator of the response rate was the number of all enrolled patients) at 4-week follow-up. For patients who experienced a bleeding response, re-bleeding was defined as the need for blood transfusion or for local salvage treatment to be performed. Blood sampling, collection of data on transfusion and local salvage treatment, and a check for adverse events were each performed at 2 weeks (± 1 week), 4 weeks (± 1 week), and 8 weeks (± 2 weeks) after enrollment (Fig. 1). After 8 weeks, data on transfusion and salvage treatment were collected monthly until 6 months after enrollment. The secondary endpoints included the proportion of patients that completed radiotherapy treatment, adverse events, overall survival, and local salvage treatment for bleeding gastric cancer. Adverse events were graded with the CTCAE version 4.0 criteria. Radiation oncologists at the participating centers discerned and recorded whether the observed adverse events were due to the radiotherapy or not. This was necessary, because patients with advanced gastric cancer frequently have some tumor-related symptoms, and chemotherapy and/or molecular targeted therapy after the radiotherapy may cause some adverse events.
Statistical analysis
The sample size was based on the precision of the estimate of a two-sided 90% confidence interval (CI) of the per-protocol (PP) response rate (i.e., the denominator of the response rate was the number of patients who completed the planned radiotherapy and were evaluable at follow-up). The PP response rate was assumed to be 70% at 4 weeks. We required 33 patients to be evaluable at 4 weeks when the desired probability of obtaining a CI ≤ ± 15% was set at 0.8. On the assumption that 45% of patients would not be evaluable at 4-week follow-up, we needed to enroll 60 patients.
Overall survival, defined as the time from enrollment until death from any cause, was estimated using the Kaplan–Meier method; median follow-up was estimated using the reverse Kaplan–Meier method [12]. Patients who were lost to follow-up without experiencing death were treated as censored on the last date they were known to be alive. Univariable and multivariable analyses with Cox proportional hazards models were performed to identify predictors of overall survival. We selected the following nine potential predictors: BED (alpha/beta = 10), bleeding response, age, sex, Palliative Prognostic Index [13], T category, M category, previous chemotherapy or molecular targeted therapy, and opioid analgesic use. In multivariable Cox regression model, any covariate with a P value < 0.10 at univariable analysis was included as explanatory variables. BED and bleeding response, the covariates of interest, were included in the multivariable analysis irrespective of the P values in univariable analysis. Bleeding response was analyzed as a time-dependent covariate [14]. Competing risks analysis was performed using proportional subdistribution hazard models (Fine-Gray model) [15]. In the Fine-Gray model, the event of interest was “bleeding response” and the competing event was “death.” In the multivariable Fine-Gray model, any of the seven covariates (age, sex, Palliative Prognostic Index, T category, M category, previous chemotherapy or molecular targeted therapy, and opioid analgesic use) with a P value < 0.10 in univariable analysis were used as explanatory variables. BED, the covariate of interest, was included in the multivariable analysis irrespective of the P value in univariable analysis. When the presence of confounding was suspected concerning some variables, the Pearson’s correlation coefficient was estimated for those variables. A univariable Cox proportional hazards model was fitted to study the effect of BED on the transition hazard [16] for transition from “response” to “re-bleeding” to investigate whether BED influences the occurrence of re-bleeding.
Multistate models are a statistical tool that allows the characterization of patients’ time course through events with a finite number of states [17]. Although the standard survival analysis only evaluates the states of a patient being alive and dead, multistate modelling can incorporate more than two states into the model, and therefore, can capture more aspects of patient history. In this study, we specified a time-inhomogeneous Markov multistate model to characterize the time course concerning treatment response, re-bleeding, and death. The model comprised three transient states (i.e., the states out of which a transition is possible) and one absorbing state (i.e., the state out of which no transition is modelled) “death” (Fig. 2). The possible transitions between the states are indicated by the arrows between the rectangles. All patients started to be followed-up at the “bleeding” state, beginning from the enrollment date. If a patient experienced a response, the patient transitioned to the state “response.” If a patient who was in the state “response” experienced re-bleeding, the patient transitioned to the state “re-bleeding.” The state “death” can be entered from any of the three transient states. State occupation probability, which is estimated in multistate models, assesses the probability that a patient is in a given state at a given time [18]. State occupation probability has a nice mathematical feature that when it is plotted against time for a given state, the area under the curve within a time interval equals an estimate of the restricted mean length of stay in that state [19]. “Restricted” implies that the duration of a patient being in a given state is calculated only up to a specific time. We estimated the restricted mean durations of response and re-bleeding up to 8 months. CIs of the restricted mean durations of response and re-bleeding were calculated by bootstrapping (1000 iterations) [19].
Multistate model. The numbers beside the arrows indicate the numbers of actual transitions between the states. In the lower diagram, the distance between two adjacent curves at a given time indicates the estimate of the probability of a patient being in the state at that time. The area of the regions demarcated by two adjacent curves up to 8 months equals the estimate of the restricted mean time spent in the state
All tests were two-tailed; P < 0.05 was considered significant. Statistical analyses were performed with R version 3.6.2. and SAS version 9.4 (SAS Institute, Cary, NC). The R package ‘mstate’ was used to estimate transition probabilities and restricted mean sojourn time in the multistate model [20].
Results
Patients
Between October 12, 2017 and September 30, 2020, 55 patients were enrolled from 15 institutions (Table 1); the median number of patients enrolled per institution was three (range 1–9 patients). Although the number of enrolled patients was less than the planned number of 60, 37 patients were evaluable in terms of the primary endpoint of the ITT response rate at 4-week follow-up. As this number of patients was larger than that of evaluable patients (33 patients) required as per our sample size calculation, we discontinued enrollment at the end of the planned enrollment period. The data cut-off date were January 15, 2021. Of the 55 enrolled patients, 54 had primary gastric cancer and 1 had postoperative recurrent gastric cancer. Nine (16%) patients had received local treatment (endoscopic therapy, surgery, or transcatheter arterial embolization) for bleeding gastric cancer at enrollment. Two patients underwent bypass surgery before radiotherapy. Before radiotherapy, 27 (49%), 36 (65%), 44 (80%), 38 (69%), and 29 patients (53%) had nausea, anorexia, fatigue, dyspnea, and abdominal pain, respectively.
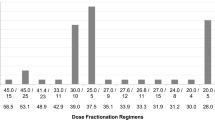
The median interval between enrollment and the initiation of radiotherapy was 2 days (range 0–9 days). Two patients did not receive the planned radiotherapy for bleeding gastric cancer (Fig. 1). Of the 53 patients who received the radiotherapy (three-dimensional radiotherapy), 50 (94%) completed the planned therapy. A median total radiation dose of 20 Gy (range 8–45 Gy) was delivered in a median of five fractions. The majority of the patients received one of the following three radiation schedules: 8 Gy in 1 fraction, 20 Gy in 5 fractions, or 30 Gy in 10 fractions.
Of the 19 patients who had not received either of chemotherapy or molecular targeted therapy before radiotherapy, 5 (26%) initiated at least one of these therapies after radiotherapy. Of the 36 patients who had received either of chemotherapy or molecular targeted therapy before radiotherapy, 11 (31%) again received at least one of these therapies after radiotherapy.
Response and re-bleeding
Of the 55 patients, 38 (69%) experienced bleeding response (Fig. 2). Of the 38 responders, 19 (50%) died and 12 (32%) experienced re-bleeding. Of the 55 enrolled patients, 45 (82%), 36 (65%), and 30 (55%) constituted the denominators of the PP response rates at 2-, 4-, and 8-week follow-up, respectively (i.e., these patients completed the planned radiotherapy and were evaluable for bleeding response). Bleeding response was observed in 26, 29, and 27 patients at 2, 4, and 8 weeks, respectively. The ITT response rates for the 55 enrolled patients were 47% (95% CI 34–61%), 53% (95% CI 39–66%), and 49% (95% CI 35–63%) at 2, 4, and 8 weeks, respectively. The PP response rates for evaluable patients were 56% (95% CI 40–70%), 78% (95% CI 61–90%), and 90% (95% CI 73–98%) at 2, 4, and 8 weeks, respectively. Observed 90% CI of the PP response rate at 4 weeks (64–89%) was narrower than the prespecified width (CI width ≤ ± 15%) in the sample size calculation. In the 12 patients who experienced re-bleeding, diagnosis was established on blood transfusion (11 patients) or re-irradiation (one patient).
In Fig. 2, the stacked probabilities of a patient being in a given state are plotted against time, beginning from the time of enrollment. The distance between two adjacent curves at any given time indicates the probability of a patient being in the state at that time. Restricted mean durations of “response” and “re-bleeding” up to 8 months, which equal the areas of the regions demarcated by two adjacent curves, were 2.3 months (95% CI 1.7–3.1 months) and 0.8 months (95% CI 0.4–1.3 months), respectively.
Predictors of survival
There were 43 observed deaths. The median follow-up was 12.1 months (95% CI 6.4 months–not estimable). The median overall survival was 3.8 months (95% CI 2.8–6.1 months). There were no missing values in any of the explanatory variables. Multivariable Cox regression analysis showed that neither BED nor bleeding response was a significant predictor of overall survival (Table 2).
Predictors of response and re-bleeding
Although univariable analysis with the Fine-Gray model showed that higher BED was associated with a higher probability of the occurrence of bleeding response, the multivariable analysis showed that BED was not a significant predictor of bleeding response (Table 3). Palliative Prognostic Index and BED were negatively correlated (Pearson’s correlation coefficient, − 0.40; CI − 0.60 to − 0.15). The univariable Cox regression model, which was used to study the effect of BED on the transition hazard for the transition from “response” to “re-bleeding” showed that BED was not significantly associated with re-bleeding (hazard ratio 0.98; 95% CI 0.92–1.04; P = 0.49).
Adverse events
Table 4 shows all the observed adverse events, including those that were judged by radiation oncologists at participating centers to be radiotherapy induced. The majority of the patients experienced mild or no adverse events attributed to radiotherapy. Grade 1, 2, 3, and ≥ 4 radiation-related adverse events were reported in 11 (20%), 9 (16%), 1 (2%), and 0 (0%) patients, respectively. Grade 2 radiation-related adverse events were nausea (3 patients; 5%), anorexia (6 patients; 11%), fatigue (6 patients; 11%), and diarrhea (1 patient; 2%); the grade 3 radiation-related adverse event was anorexia (1 patient; 2%). One patient died of cerebral infarction 14 days after the radiotherapy for gastric cancer (8 Gy in 1 fraction), and this event was recorded as unrelated to radiotherapy by the radiation oncologist.
Discussion
We found that in evaluable patients, PP response rate increased to 90% during the 8-week follow-up. Palliative radiotherapy for bleeding gastric cancer should be able to improve tumor-related anemia in a high proportion of patients. More than half of the bleeding response events may occur within 2 weeks, but it may take 8 weeks for radiotherapy to show its full effect. Appropriate timing of the assessment of effectiveness is yet to be determined in palliative radiotherapy for gastric cancer. When to assess treatment response in studies of palliative radiotherapy depends on both the time course of response and the attrition of patients [21]. We recommend that the primary assessment of response should be performed at 4 weeks in studies on palliative radiotherapy for gastric cancer, also considering the high hazard of death of these patients. Mainly because death frequently occurred, the ITT response rate remained approximately 50% throughout the 8-week follow-up period. At least 1 of the 2 patients for whom palliative radiotherapy was planned received benefit from the treatment.
Palliative radiotherapy appeared to only improve anemia and not survival based on our finding that bleeding response was not associated with better survival. Although a few studies have demonstrated that responders to radiotherapy had better survival than non-responders, they appear to have treated bleeding response as a baseline covariate [9, 22]. Response may or may not occur after the initiation of follow-up. Treating such time-dependent covariate as baseline information leads to time-dependent bias, which artificially inflates the protective effect and damps down the harmful effect of that covariate [14, 23]. We also found that BED was not significantly associated with survival; this finding is in line with past studies that did not find dose–response relationship in terms of survival [5, 24, 25]. Absence of dose–response relationship further supports the hypothesis that radiotherapy does not significantly improve survival of these patients.
We did not find a significant association between BED and bleeding response. In the multivariable analysis with the Fine-Gray model, BED lost its significance found in univariable analysis; this may be because the Palliative Prognostic Index was a confounder of the association between BED and bleeding response. The reason why Palliative Prognostic Index and BED were negatively correlated might be that the treating radiation oncologists may have tended to choose shorter radiation schedules and accordingly lower BEDs for patients with poorer prognosis. The apparent relation between BED and bleeding response in univariable analysis may be because patients who received radiotherapy with higher BEDs tended to have lower Palliative Prognostic Index scores and thus lower probability of death, and accordingly, tended to have more chance of experiencing bleeding response. Some studies found a significant association between higher radiation dose and higher frequency of bleeding response by univariable [26] and multivariable analyses [27]; however, other studies did not find such associations [5, 22]. The Palliative Prognostic Index [13] would be an important covariate to adjust when analyzing the association between radiation dose and bleeding response, and our study is an important addition to the literature. We also did not find a significant association between BED and re-bleeding. Because re-bleeding was only observed in 12 patients and the CI was wide, we could not reach any firm conclusions regarding this association.
Our results suggest that dose escalation beyond 30 Gy in 10 fractions with the intent to prolong the duration of response may provide limited benefit. Death, and not re-bleeding, was the predominant cause of the end of response in the present study where most of the patients received radiotherapy with a total radiation dose ≤ 30 Gy. Moreover, we found that the restricted mean duration of re-bleeding was only 0.8 months; i.e., patients who experienced re-bleeding, on average, died shortly after the occurrence of re-bleeding. The relatively low radiation doses utilized in our study may be reasonable options in palliative radiotherapy for bleeding gastric cancer.
Causes of the recorded adverse events are difficult to specify. This is because many patients had some symptoms at baseline, and chemotherapy and/or molecular targeted therapy, performed between enrollment and the assessment of adverse events, may have caused some of the recorded events. The majority of the patients experienced mild or no radiation-related adverse events as judged by participating centers’ radiation oncologists.
There were limitations to our study. First, the sample size was not large enough to assess the influence of BED on re-bleeding. Further studies are warranted that examine the association between radiation dose and the duration of response. Second, assessment of re-bleeding was based on requirement of blood transfusion and local salvage treatment for bleeding, and not on hemoglobin levels. As patients who receive palliative radiotherapy for bleeding gastric cancer, effectively, have a poor prognosis, we could not plan blood sampling in the assessment of re-bleeding from the standpoint of feasibility. Third, dose prescription was determined at the discretion of the treating radiation oncologist. Some confounders, including unmeasured ones, may not have been adjusted in multivariable analyses for the relation between BED and endpoints. Although these analyses were hypothesis-generating, currently, we have to use best available evidence to guide our practice. We think our study is a valuable contribution to the literature.
In summary, in the largest prospective and first multicenter study on palliative radiotherapy for bleeding gastric cancer, we demonstrated a high PP response rate in patients treated with relatively low radiation doses. Palliative radiotherapy may be highly effective in improving anemia with mild toxicities. The frequent occurrence of death from shortly after enrollment lowered the ITT response rate and shortened the duration of response. The strategy for palliative intervention for bleeding gastric cancer should take into account the limited survival of these patients. We did not find any evidence that the use of higher BEDs would lead to patients’ benefits in terms of survival, response, or re-bleeding. The analysis on the association between radiation dose and re-bleeding was limited by the small number of events, and further larger studies investigating the optimal radiation dose, preferably randomized controlled trials, are warranted.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Warschkow R, Baechtold M, Leung K, Schmied BM, Nussbaum DP, Gloor B, et al. Selective survival advantage associated with primary tumor resection for metastatic gastric cancer in a Western population. Gastric Cancer. 2018;21:324–37.
Dittmar Y, Rauchfuss F, Goetz M, Jandt K, Scheuerlein H, Heise M, et al. Non-curative gastric resection for patients with stage 4 gastric cancer–a single center experience and current review of literature. Langenbecks Arch Surg. 2012;397:745–53.
Sheibani S, Kim JJ, Chen B, Park S, Saberi B, Keyashian K, et al. Natural history of acute upper GI bleeding due to tumours: short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther. 2013;38:144–50.
Sugita H, Sakuramoto S, Mihara Y, Matsui K, Nishibeppu K, Ebara G, et al. Verification of the utility of palliative radiotherapy for hemostasis of gastric cancer bleeding: a case control study. J Gastrointest Cancer. 2021. https://doi.org/10.1007/s12029-021-00632-y.
Kawabata H, Hitomi M, Motoi S. Management of bleeding from unresectable gastric cancer. Biomedicines. 2019;7:54.
Viani GA, Arruda CV, Hamamura AC, Faustino AC, Danelichen AFB, Matsuura FK, et al. Palliative radiotherapy for gastric cancer: is there a dose relationship between bleeding response and radiotherapy? Clinics (Sao Paulo). 2020;75:e1644.
Kosugi T, Shikama N, Saito T, Nakamura N, Nakura A, Harada H, et al. A Nationwide survey in Japan of palliative radiotherapy for bleeding in gastrointestinal and genitourinary tumor patients. World J Oncol. 2016;7:29–33.
Tey J, Zheng H, Soon YY, Leong CN, Koh WY, Lim K, et al. Palliative radiotherapy in symptomatic locally advanced gastric cancer: a phase II trial. Cancer Med. 2019;8:1447–58.
Tanaka O, Sugiyama A, Omatsu T, Tawada M, Makita C, Matsuo M. Hemostatic radiotherapy for inoperable gastric cancer: a pilot study. Br J Radiol. 2020;93:20190958.
Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6.
Morita T, Tsunoda J, Inoue S, Chihara S. The Palliative Prognostic Index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer. 1999;7:128–33.
Beyersmann J, Wolkewitz M, Schumacher M. The impact of time-dependent bias in proportional hazards modelling. Stat Med. 2008;27:6439–54.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430.
Lange JM, Hubbard RA, Inoue LYT, Minin VN. A joint model for multistate disease processes and random informative observation times, with applications to electronic medical records data. Biometrics. 2015;71:90–101.
Andersen PK, Pohar PM. Inference for outcome probabilities in multi-state models. Lifetime Data Anal. 2008;14:405–31.
Beyersmann J, Putter H. A note on computing average state occupation times. Demogr Res. 2014;30:1681–96.
de Wreede L, Fiocco M, Putter H. mstate: An R package for the analysis of competing risks and multi-state models. J Stat Softw. 2011;38:1–30.
Li KK, Hadi S, Kirou-Mauro A, Chow E. When should we define the response rates in the treatment of bone metastases by palliative radiotherapy? Clin Oncol (R Coll Radiol). 2008;20:83–9.
Tey J, Choo BA, Leong CN, Loy EY, Wong LC, Lim K, et al. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine (Baltimore). 2014;93:e118.
Beyersmann J, Gastmeier P, Wolkewitz M, Schumacher M. An easy mathematical proof showed that time-dependent bias inevitably leads to biased effect estimation. J Clin Epidemiol. 2008;61:1216–21.
Kim MM, Rana V, Janjan NA, Das P, Phan AT, Delclos ME, et al. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008;47:421–7.
Chaw CL, Niblock PG, Chaw CS, Adamson DJ. The role of palliative radiotherapy for haemostasis in unresectable gastric cancer: a single-institution experience. Ecancer Med Sci. 2014;8:384.
Hashimoto K, Mayahara H, Takashima A, Nakajima TE, Kato K, Hamaguchi T, et al. Palliative radiation therapy for hemorrhage of unresectable gastric cancer: a single institute experience. J Cancer Res Clin Oncol. 2009;135:1117–23.
Lee YH, Lee JW, Jang HS. Palliative external beam radiotherapy for the treatment of tumor bleeding in inoperable advanced gastric cancer. BMC Cancer. 2017;17:541.
Acknowledgements
We thank the patients who participated in this study, their caregivers, and the investigators and clinical research staff from the study centers.
Funding
This work was supported by the Japanese Radiation Oncology Study Group (JROSG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saito, T., Kosugi, T., Nakamura, N. et al. Treatment response after palliative radiotherapy for bleeding gastric cancer: a multicenter prospective observational study (JROSG 17-3). Gastric Cancer 25, 411–421 (2022). https://doi.org/10.1007/s10120-021-01254-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01254-w