Abstract
Background and aims
A drawback of endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) is the development of metachronous gastric cancer (MGC). While MGC after ESD for differentiated-type (D-) EGC was well understood, little is known about MGC occurring after ESD for undifferentiated-type (UD-) EGC, because ESD had not been indicated. We evaluated the incidence and treatment outcomes of MGC after ESD of UD-EGC.
Methods
This study is a post hoc analysis of JCOG1009/1010, a multicenter trial to evaluate the efficacy and safety of ESD for UD-EGC. The patients who underwent curative ESD of index solitary UD-EGC were analyzed. Surveillance endoscopy was performed biannually for the first 3 years and thereafter annually. We assessed the time to MGC occurrence after ESD, lesion characteristics, and treatment outcomes of MGC. Time to MGC occurrence was estimated by cumulative incidence function, with death and total gastrectomy as competing risks.
Results
A total of 198 patients were included in this study. During a median follow-up period of 5.8 years, 4 patients (2%) developed MGC. Median time to MGC occurrence was 4.5 years (range: 3.1–5.4). Five-year cumulative incidence of MGC was 1.0% (95% CI: 0.2–3.3%). Two MGCs were histologically D-EGC, and the remaining two were UD-EGC. The median tumor size of MGCs was 1.0 cm (range: 0.7–1.7), and the depth of invasion (M/SM1/SM2) was 2/1/1, respectively. Three patients achieved curative resection with repeated ESD.
Conclusions
MGC does not occur commonly after curative ESD of UD-EGC, and repeated ESD could contribute to stomach preservation.
Similar content being viewed by others
Introduction
Endoscopic resection (ER) is a minimally invasive treatment for early gastric cancer (EGC). Among several methods of ER, endoscopic submucosal dissection (ESD) has allowed for en-bloc and curative resection regardless of lesion size and location [1, 2]. Also, ESD can preserve the stomach and maintain the quality of life in those who achieve curative ER. Several retrospective and prospective studies have demonstrated favorable long-term ESD outcomes in patients with differentiated-type EGC (D-EGC) undergoing curative ER [3,4,5]. Thus, ESD is now accepted as a standard of care for D-EGC [6].
In contrast, ESD had not been traditionally indicated for undifferentiated-type EGC (UD-EGC) because of a lack of evidence in long-term outcomes in prospective studies [6, 7]. However, we recently reported that, in a multicenter, non-randomized confirmatory trial of ESD to expand its indication for UD-EGC (JCOG1009/1010), ESD of T1a UD-EGC ≤ 2 cm without ulceration provided comparable survival to those that underwent surgery with lymph node dissection [8].
A drawback of ER for EGC when compared with gastrectomy is the development of metachronous gastric cancer (MGC) in the preserved stomach. It could have a possibility of the requirement of gastrectomy or gastric cancer death due to MGC. Several studies have showed that the three-year and five-year cumulative incidences of MGC occurring after curative ER were 5.9% [9, 10] and 9.5–14.0% [11, 12], respectively, with an approximate annual incidence of 2.5–4% [9, 11,12,13]. Generally, MGCs can be curatively treated with ESD, allowing for preservation of the stomach function. However, the previous studies included patients with D-EGC mostly because UD-EGCs had been mainly treated by gastrectomy with lymph node dissection. Although MGC might develop even after curative ESD of UD-EGC, little is known about the actual incidence of MGC and treatment outcomes of MGC in patients with UD-EGC. We aimed to evaluate the incidence and treatment outcomes of MGC after curative gastric ESD of UD-EGC by a post hoc analysis of JCOG1009/1010.
Patients and methods
Patients
This study was a post hoc analysis of data collected during JCOG1009/1010 which aimed to evaluate the efficacy and safety of ESD within an indication specified for UD-EGC [8]. The key inclusion criteria of the JCOG1009/1010 were as follows: (1) histologically proven index solitary gastric UD-type adenocarcinoma (poorly differentiated adenocarcinoma or signet-ring cell carcinoma) on biopsy, (2) endoscopically diagnosed T1a tumor ≤ 2 cm in size without ulceration and (3) absence of lymph node and distant metastasis (cN0M0) in computed tomography, (4) no prior gastrectomy and no reconstructive surgery using the stomach [8]. Index EGC was defined as an EGC which were initially treated during the study period.
Amongst those who met the aforementioned inclusion criteria, this post hoc analysis included patients who underwent curative ESD of UD-type, which was defined as histologically proven T1a UD-EGC without ulceration or lymphovascular invasion ≤ 2 cm with free lateral and deep margin. Undifferentiated dominant type (e.g., a mixed histological type, such as poorly to moderately differentiated adenocarcinoma) was also included as UD-EGC in this study. Those who undergoing curative ESD of D-type, which was defined as well-differentiated tubular adenocarcinoma, moderately differentiated tubular adenocarcinoma, and papillary adenocarcinoma, and those with any non-curative ESD were excluded from the analysis. The JCOG1009/1010 study protocol was reviewed and approved by the Protocol Review Committee of JCOG in Dec 2010 followed by the institutional review board at each participating institution prior to initiation of the study. Written informed consent including for the secondary use of the data was obtained from the enrolled patients at the registration to JCOG1009/1010. The JCOG1009/1010 was conducted in accordance with the precepts established in the Declaration of Helsinki, and registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000004995).
Patients follow-up after ESD of the index EGC
Surveillance endoscopy after ESD of the index EGC was performed biannually for the first three years and thereafter annually. If a suspicious lesion was detected during surveillance endoscopy, the targeted biopsy specimen was taken from the lesion. MGC was defined as a histologically-proven gastric cancer located in an area apart from the index EGC scar, more than 1 year after ESD [10, 14]. Histological type, tumor size, depth of invasion, and the presence of ulceration were estimated and recorded during a surveillance endoscopy or on an additional preoperative endoscopy. Magnifying endoscopy and endoscopic ultrasound were used if clinically necessary at the discretion of each institution.
Treatment strategy and histological diagnosis of MGC
Treatment for MGC was same as for other EGC and was performed according to Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3) [15]. The resected specimens were assessed by histopathology after being fixed in 10% formalin. Histological mapping was performed after the specimens were serially sectioned at 2 mm intervals for endoscopic specimens and 5 mm intervals for surgical specimens. Certified pathologists at each institution assessed the histological type, macroscopic appearance, tumor size, depth of invasion, presence of ulceration, lymphovascular invasion, and margins, according to the Japanese classification of gastric carcinoma [16].
In the case of ER, en-bloc resection was defined when the entire lesion was located within one piece regardless of histological tumor margin. Complete resection was defined as en-bloc resection with histological free lateral and vertical margins. Curative resection was defined as complete resection without lymphovascular invasion and: (a) D-type mucosal cancer without ulceration regardless of tumor size, or (b) D-type mucosal cancer ≤ 3 cm in diameter with ulceration tumor size, or (c) D-type minute submucosal cancers (< 500 μm from the muscularis mucosae) ≤ 3 cm, or (d) UD-type mucosal cancer ≤ 2 cm without ulceration, according to Japanese gastric cancer treatment guidelines 2010 (ver. 3) [15]. The mixed histological type of the resected specimens was determined by major histological features of the resected specimen. Additional surgery was recommended for all other non-curative resections.
Data collection
Baseline patient and lesion characteristics of the study subjects were obtained from the original database of JCOG1009/1010. The data on MGC occurrence, clinical and histological characteristics, and treatment outcomes of MGCs were additionally collected from medical records of each institution. Moreover, baseline gastric mucosal atrophy and Helicobacter pylori (H. pylori) infection status at the time of the index EGC diagnosis and smoking have been reported to be positively associated with MGC occurrence. The status of H. pylori infection was defined as follows: (a) positive if any H. pylori infection test was positive, (b) negative if any H. pylori infection test(s) were negative and there was no history of H. pylori eradication, (c) Post-eradication if successful H. pylori eradication was confirmed. The kinds and number of tests to detect H. pylori infection were determined at the discretion of each institution. Statin and non-steroidal anti-inflammatory drugs (NSAIDs) use have been reported to be negatively associated with MGC occurrence. These factors were therefore retrospectively collected from medical record at each institution [17,18,19,20]. Baseline gastric mucosal atrophy was assessed endoscopically according to the Kimura-Takemoto classification, which correlates well with the histologic degree of atrophic gastritis [21]. Gastric mucosal atrophy was divided into three categories: none (closed type 0 and I), mild (closed type II and III), and severe (open type I–III).
Statistical analysis
The time to event of MGC was calculated as the interval between the date of ESD of the index EGC and the date of detection of MGC. The annual proportion of MGC was estimated using cumulative incidence function, given the competing risk of those who died of other causes or underwent total gastrectomy. Risk factors for the development of MGC were investigated by Cox proportional hazards regression models. A p value of < 0.05 was considered significant. Statistical analyses were performed by a biostatistician of the JCOG Data Center (J.M) using SAS version 9.4.
Results
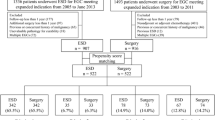
A total of 346 patients were enrolled in JCOG1009/1010, and ESD was performed in 344 patients. Among them, we excluded 42 patients with curative resection of D-type, 95 with non-curative resection, 1 UD-EGC with indeterminate curability owing to emergency surgery, and 8 with no malignancy in ESD specimen (Fig. 1). Finally, 198 patients with curative resection of UD-EGC were selected for this study. Among them, 195 patients were eligible in JCOG1009/1010, and 3 were ineligible but achieved curative ESD resection of UD-EGC and could be followed-up in accordance with the study protocol.
Baseline patient’s characteristics are shown in Table 1. Ninety-six patients were male, and 102 were female with the median age of 60 years (range: 23–80). Sixty-four patients had no baseline gastric atrophy, 64 had mild, and 70 had severe gastric atrophy at the time of the index EGC diagnosis. Eighty-six patients had H. pylori infection, 59 did not have H. pylori infection, and 26 succeeded for H. pylori eradication before ESD of the index EGC. The data of H. pylori infection were missing in 27 patients. Among 86 patients with H. pylori infection, H. pylori eradication after the index ESD was successful in 64 patients, unsuccessful in 3 patients, not performed in 14 patients, and unknown in 5 patients. Thus, 17 patients remained positive for H. pylori infection after the index ESD, 59 were negative, 90 were eradicated, and the data were missing in 32 patients. The characteristics of index EGC are shown in Table 2.
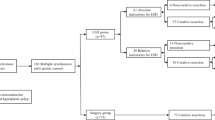
During a median follow-up period of 5.8 years (range: 0.2–7.2), no patients died of gastric cancer, as previously reported, although one died from other cause [8]. Four patients developed MGCs during the follow-up period. Median time to MGC occurrence was 4.5 years (range: 3.1–5.4). Five-year cumulative incidence MGC was 1.0% (95% CI: 0.2–3.3%) (Fig. 2). All lesions were macroscopically Type 0-IIc and resected with ESD. Two of four MGCs were histologically D-EGC, and two were UD-EGC. The median tumor size of MGCs was 1.0 cm (range: 0.7–1.7). As for the depth of invasion, two MGCs were mucosal cancer, one was minute submucosal cancer (< 500 μm from the muscularis mucosa), and the remaining one was deep submucosal cancer (≥ 500 μm from the muscularis mucosa). Three out of the four patients achieved curative resection with ESD (Tables 3, 4).
In terms of risk factors associated with MGC occurrence, NSAIDs use was significantly more common in patients with MGC (Table 5). However, multivariable Cox proportional hazards regression models were not carried out because of the small number of events.
Discussion
This study showed that 5-year cumulative incidence of MGC after curative ESD of UD-EGC was 1.0% (95% CI: 0.2–3.3%). We believe that this data was clinically reliable, because it was based on the data of multicenter clinical trial (JCOG1009/1010), while most previous studies were single-center retrospective studies with the data of lost to follow-up and indeterminate surveillance endoscopy [11, 22,23,24,25,26,27,28]. To the best of our knowledge, this is the first report on MGC occurrence following a predetermined periodic surveillance protocol. We believe that these data provide an actual incidence of MGC after curative ESD of UD-EGC.
The 3-year and 5-year cumulative incidences of MGC after D-EGC undergoing curative ER were reported to be 5.9% [9, 10] and 9.5–14.0% [11, 12], respectively. The cumulative incidence of MGC linearly increased over time with an approximate annual incidence of 2.5–4% [9, 11,12,13]. In contrast, this study demonstrated that the 5-year cumulative incidence of MGC was 1.0% (95% CI: 0.2–3.3%). Thus, MGC occurrence after ESD of UD-EGC seemed to be lower than that of D-EGC. Ishioka et al. reported that the 5-year cumulative incidences of MGC in the UD-EGC and D-EGC group were 3.5 and 20.8%, respectively, and the difference was significant (p < 0.01) [28]. This data, together with our study, supported the low MGC incidence after curative ESD of UD-EGC.
The reasons for the low MGC occurrence rate in the UD-EGC could be explained by the difference in gastric atrophy and following intestinal metaplasia. UD-gastric cancer generally develops during the progression of atrophic gastritis, and the development of D-gastric cancer is closely related to severe atrophic gastritis accompanying intestinal metaplasia caused by persistent H. pylori infection. Park et al. showed that atrophic gastritis with intestinal metaplasia was also an independent risk factor of synchronous or metachronous gastric cancer in Cox hazard model [25]. Maehata et al. revealed that severe mucosal atrophy was an independent risk factor for the development of MGC [29]. In our study, despite unavailable data of intestinal metaplasia, the proportion of severe gastric atrophy in the baseline characteristics was 35% (70/198). On the other hand, the proportions in previous studies that included mostly patients with D-EGC were 55–62% [29, 30]. Another reason to explain the low incidence of MGC in this study was specific background factors at index EGCs. Some retrospective studies which mostly included D-EGCs showed that older age, male, and multiple index EGCs at the time of the index ESD were also the independent risk factors of MGC occurrence [9, 31]. JCOG1009/1010 included only solitary UD-EGC. Besides, because the patients with UD-EGC were characterized as female-dominant and younger than other previous studies, these factors could also explain the low MGC.
In terms of other known risk factors for MGC occurrence, this study collected H. pylori infection status, NSAIDs and statin use, and smoking. Of these, H. pylori infection is considered one of the most important risk factors for gastric cancer [32]. Interestingly, in terms of the comparison between H. pylori infected and uninfected before ESD, Ishioka et al. reported that all patients who developed MGCs after ESD of UD-EGC were H. pylori-infected, and there were no MGCs in patients without H. pylori infection, although the discussion is still controversial [28]. Some randomized controlled trials (RCTs) concluded that H. pylori eradication effectively reduced MGC occurrence after ER for D-EGC [33, 34]. The main study subjects of these RCTs, were patients with D-EGC, and the efficacy of H. pylori eradication for MGC reduction in patients with UD-EGC remains unknown. NSAIDs use, which was reported to be negatively associated with MGC occurrence [20], was significantly more common in patients with MGC in the log-rank test. Possibly, the retrospective data collection with missing data might explain the observed inverse relationship.
Previous studies included index D-EGC suggested annual or biannual life-long surveillance endoscopy to achieve early detection of MGC [11, 12, 35]. In JCOG1009/1010, the surveillance endoscopy was performed biannually for the first 3 years and thereafter annually. This surveillance protocol could help early detection of MGC following curative ESD of UD-EGC. Given the MGC characteristics and the curability of ESD for MGC, the interval of surveillance endoscopy should be carefully determined despite the low incidence of MGC, because repeated ESD needs to be strictly indicated for UD-type MGC, limited to cT1a ≤ 2 cm in size without ulceration. Further investigations are warranted to establish an optimal surveillance protocol after ESD for UD-EGC.
There were several limitations to this study. First, some additional data, such as the status of H. pylori infection, smoking, and NSAIDs use were retrospectively analyzed, and there were some missing data. Specifically, H. pylori infection test and eradication were not determined in the protocol of JCOG1009/1010, and they were carried out at the discretion of each institution. As some data were missing, it was challenging to determine H. pylori-infected or -uninfected in this post hoc analysis. Second, the original data included only patients with an index solitary UD-EGC, and excluded those with multiple EGCs as discussed above. Third, the number of events was small. These limitations made it difficult to perform a comprehensive risk analysis of MGC with multivariable Cox proportional hazards regression models in patients with index UD-EGC. To clarify the incidence, risk factors, and treatment outcomes of MGCs, further large-scale prospective study is warranted. A prospective study (J-WEB-EGC) was conducted to evaluate the long-term outcomes of ESD for EGC in Japan. This web-based large cohort study enrolled 9616 patients with both UD- and D-EGC from 41 institutions over 2 years [2, 36]. The long-term outcomes of this study will provide more detailed and convincing data to stratify the risk factors associated with MGC including H. pylori eradication and the histology at the index EGC, which may help the establishment of a surveillance program for MGC after curative endoscopic resection of UD-EGC.
In conclusion, MGC does not occur commonly after curative ESD of UD-EGC and repeated ESD could contribute to stomach preservation. Thus, ESD could be a standard treatment for cT1a UD-EGC without ulceration ≤ 2 cm from the MGC management point of view.
References
Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9(4):262–70.
Suzuki H, Takizawa K, Hirasawa T, Takeuchi Y, Ishido K, Hoteya S, et al. Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: ‘Real-world evidence’ in Japan. Dig Endosc. 2019;31(1):30–9.
Hasuike N, Ono H, Boku N, Mizusawa J, Takizawa K, Fukuda H, et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer. 2018;21(1):114–23.
Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19(1):198–205.
Tanabe S, Ishido K, Matsumoto T, Kosaka T, Oda I, Suzuki H, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a multicenter collaborative study. Gastric Cancer. 2017;20(Suppl 1):45–52.
Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric cancer 2020. Epub 2020/02/16. Doi: https://doi.org/10.1007/s10120-020-01042-y.
Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28(1):3–15.
Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, et al. A non-randomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer. 2020;24(2):479–491. https://doi.org/10.1007/s10120-020-01134-9 (Epub 2020/11/09).
Hahn KY, Park CH, Lee YK, Chung H, Park JC, Shin SK, et al. Comparative study between endoscopic submucosal dissection and surgery in patients with early gastric cancer. Surg Endosc. 2018;32(1):73–86.
Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, et al. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer. 2006;9(2):93–8.
Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Nakajima T, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy. 2015;47(12):1113–8.
Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62(10):1425–32.
Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37(10):990–3.
Nishida T, Tsujii M, Kato M, Hayashi Y, Akasaka T, Iijima H, et al. Endoscopic surveillance strategy after endoscopic resection for early gastric cancer. World J Gastrointest Pathophysiol. 2014;5(2):100–6.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver.3). Gastric Cancer. 2011;14(2):113–23.
Japanese classification of gastric carcinoma: 3rd English edition. Gastric cancer. 2011;14(2):101–12.
Ami R, Hatta W, Iijima K, Koike T, Ohkata H, Kondo Y, et al. Factors associated with metachronous gastric cancer development after endoscopic submucosal dissection for early gastric cancer. J Clin Gastroenterol. 2017;51(6):494–9.
Ford AC. Chemoprevention for gastric cancer. Best Pract Res Clin Gastroenterol. 2011;25(4–5):581–92.
Gong EJ, Ahn JY, Jung HY, Lim H, Choi KS, Lee JH, et al. Risk factors and clinical outcomes of gastric cancer identified by screening endoscopy: a case-control study. J Gastroenterol Hepatol. 2014;29(2):301–9.
Ma Z, Wang W, Jin G, Chu P, Li H. Effect of statins on gastric cancer incidence: a meta-analysis of case control studies. J Cancer Res Ther. 2014;10(4):859–65.
Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1(3):87–97.
Ahn JY, Park HJ, Park YS, Lee JH, Choi KS, Jeong KW, et al. Endoscopic resection for undifferentiated-type early gastric cancer: immediate endoscopic outcomes and long-term survivals. Dig Dis Sci. 2016;61(4):1158–64.
Kim YY, Jeon SW, Kim J, Park JC, Cho KB, Park KS, et al. Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc. 2013;27(12):4656–62.
Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44(2):122–7.
Park CH, Kim EH, Kang JH, Chung H, Park JC, Shin SK, et al. Low incidence of synchronous or metachronous tumors after endoscopic submucosal dissection for early gastric cancer with undifferentiated histology. PLoS One. 2016;11(1):e0147874.
Yang HJ, Kim SG, Lim JH, Choi JM, Oh S, Park JY, et al. Surveillance strategy according to age after endoscopic resection of early gastric cancer. Surg Endosc. 2018;32(2):846–54.
Lim H, Lee JH, Park YS, Na HK, Ahn JY, Kim DH, et al. A single-center experience of endoscopic resection for early gastric cancer with lymphoid stroma. J Gastric Cancer. 2018;18(4):400–8.
Ishioka M, Yoshio T, Miyamoto Y, Namikawa K, Tokai Y, Yoshimizu S, et al. Incidence of metachronous cancer after endoscopic submucosal dissection: a comparison between undifferentiated-type and differentiated-type early gastric cancer. Gastrointest Endosc. 2020;93(3):557-564.e1. https://doi.org/10.1016/j.gie.2020.06.067 (Epub 2020/07/06).
Maehata Y, Nakamura S, Fujisawa K, Esaki M, Moriyama T, Asano K, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75(1):39–46.
Mori G, Nakajima T, Asada K, Shimazu T, Yamamichi N, Maekita T, et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large-scale, multicenter cohort study in Japan. Gastric Cancer. 2016;19(3):911–8.
Kobayashi M, Narisawa R, Sato Y, Takeuchi M, Aoyagi Y. Self-limiting risk of metachronous gastric cancers after endoscopic resection. Dig Endosc. 2010;22(3):169–73.
Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114(6):1169–79.
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–7.
Choi JM, Kim SG, Choi J, Park JY, Oh S, Yang HJ, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc. 2018;88(3):475-85.e2.
Min BH, Kim ER, Kim KM, Park CK, Lee JH, Rhee PL, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2015;47(9):784–93.
Oda I, Shimazu T, Ono H, Tanabe S, Iishi H, Kondo H, et al. Design of Japanese multicenter prospective cohort study of endoscopic resection for early gastric cancer using Web registry (J-WEB/EGC). Gastric Cancer. 2012;15(4):451–4.
Acknowledgements
This supplementary analysis used the collected data of JCOG1009/1010 that was supported by Grants-in-Aid for Cancer Research (20S-3, 20S-6, 26-A-4), the National Cancer Center Research and Development Fund (23-A-16, 23-A-19, 26-A-4, 29-A-3 and 2020-J-3) and Health and Labour Science Research Grant for Clinical Cancer Research (22-021) from the Ministry of Health, Labour and Welfare, Japan. Participating institutions: Gastrointestinal Endoscopy Study Group of the Japan Clinical Oncology Group (JCOG); Iwate Prefectural Central Hospital; Iwate Medical University Hospital; Yamagata Prefectural Central Hospital; Ibaraki Prefectural Central Hospital; Tochigi Cancer Center; National Cancer Center Hospital East; Asahi Hospital; Chiba Cancer Center; National Cancer Center Hospital; Showa University Hospital; Cancer Institute Hospital; Toranomon Hospital; Kanagawa Cancer Center; Yokohama Municipal Citizen’s Hospital; Kitasato University East Hospital; Yokohama City University Medical Center; Ishikawa Prefectural Central Hospital; Saku Central Hospital Advanced Care Center; Shizuoka Cancer Center Hospital; Aichi Cancer Center, Aichi. Aichi Cancer Center Aichi Hospital; Kyoto University Graduate School of Medicine: Osaka International Cancer Institute; Osaka City General Hospital; Kobe University Hospital; Hyogo Cancer Center; Shikoku Cancer Center; Kochi Health Sciences Center; Sano Hospital; Hiroshima City Hospital; NTT Medical Center Tokyo; Niigata University; Sendai Medical Center; Miyagi Cancer Center; Tokyo Metropolitan Bokutoh Hospital; Niigata Cancer Center Hospital; Tsubame Rosai Hospital; Toyama Prefectural Central Hospital; Gifu Municipal Hospital; Shizuoka General Hospital; Kyoto Medical Center; Japanese Red Cross Kyoto Daini Hospital; Osaka University; Kinki University; Osaka National Hospital; Osaka Medical College; Sakai Municipal Hospital; Hyogo College of Medicine; Itami City Hospital; Tenri Hospital; Wakayama Medical University; Hiroshima City Asa Hospital; Oita University Hospital; Toyonaka Municipal Hospital; Keiyukai Sapporo Hospital. In addition, we thank Dr. Saowanee Ngamruengphong (Division of Gastroenterology and Hepatology, Johns Hopkins Medicine, Baltimore, USA) for editing a draft of the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Seiichiro Abe: Boston Scientific (personal fees), AstraZeneca (personal fees), Takeda Pharmaceutical (personal fees), Olympus (personal fees and research funding), FUJIFILM (personal fees and research funding), Kohei Takizawa: Takeda Pharmaceutical (personal fees), Otsuka Pharmaceutical (personal fees), EA Pharma (personal fees), Boston Scientific (personal fees), AstraZeneca (personal fees), Taiho Pharmaceutical (personal fees), Ichiro Oda: Otsuka Pharmaceutical (personal fees), Taiho Pharmaceutical (personal fees), Takeda Pharmaceutical (personal fees), Olympus (personal fees), FUJIFILM (Research funding), Junki Mizusawa: no conflict of interest, Tomohiro Kadota: no conflict of interest, Hiroyuki Ono: Otsuka Pharmaceutical (personal fees), Daiichi-Sankyo (personal fees), EA Pharma (personal fees), Boston Scientific (personal fees), AstraZeneca (personal fees), Olympus (personal fees), Noriaki Hasuike: No conflict of interest, Tomonori Yano: Olympus (personal fees and research funding), FUJIFILM (research funding), Yoshinobu Yamamoto: No conflict of interest, Yusuke Horiuchi: Olympus (personal fees), Kaken Pharmaceutical Co.,Ltd. (personal fees), Shinji Nagata: No conflict of interest, Takaki Yoshikawa: Taiho Pharmaceutical (personal fees), Chugai Pharmaceutical (personal fees), Ono Pharmaceutical (personal fees), Bristol-Myers Squibb (personal fees), MSD (personal fees), Daiichi Sankyo (personal fees), Eli Lilly (personal fees), Pfizer (personal fees), Johnson and Johnson (personal fees), Covidien (personal fees), Olympus (personal fees), TERUMO (personal fees), Nihon Kayaku (personal fees), Masanori Terashima: Taiho Pharmaceutical (personal fees), Chugai Pharmaceutical (personal fees), Ono Pharmaceutical (personal fees), Bristol-Myers Squibb (personal fees), Yakult Honsha (personal fees), Takeda Pharmaceutical (personal fees), Eli Lilly (personal fees), Pfizer (personal fees), Daiichi Sankyo (personal fees), Manabu Muto: Olympus (research funding), Ono Pharmaceutical (research funding).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abe, S., Takizawa, K., Oda, I. et al. Incidence and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection of undifferentiated-type early gastric cancer: Japan Clinical Oncology Group study—post hoc analysis of JCOG1009/1010. Gastric Cancer 24, 1123–1130 (2021). https://doi.org/10.1007/s10120-021-01183-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01183-8