Abstract
Background
Whether splenectomy for splenic hilar lymph node (No. 10) dissection in type 4 gastric cancer involving the greater curvature is necessary is not established. Patients with type 4 gastric cancer often experience peritoneal relapse, despite curative surgery, and total gastrectomy with splenectomy is frequently associated with infectious complications.
Method
Patients with cT2–T4 gastric cancer in the upper or middle third of the stomach, or both, involving the greater curvature who underwent R0 total gastrectomy with splenectomy between 2006 and 2016 were selected. Clinicopathological findings, postoperative complications, the incidence of lymph node metastasis, and the therapeutic value index of each station were compared between type 4 and non-type 4 gastric cancer.
Results
We enrolled 50 patients with type 4 and 60 with non-type 4. The former had a significantly higher proportion of the undifferentiated type and larger and deeper tumors. The overall incidence of Grade III or higher complications was 20.9%. The incidence of No. 10 metastasis was 26.0% in type 4 and 31.7% in non-type 4. Although the therapeutic value index of the No. 10 was 13.7 in type 4 and 15.0 in non-type 4, the index of type 4 ranked just below several peri-gastric stations and seventh, while that in non-type 4 ranked second.
Conclusion
Splenectomy for No. 10 dissection may be oncologically valid for type 4 gastric cancer involving the greater curvature. A safer procedure for No. 10 dissection should be established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Borrmann classification system was established in 1926. Subsequently, the gross type of gastric cancer has usually been classified into type 0, 1, 2, 3, or 4. The incidence of type 4 gastric cancer is 13.2–16.7% of advanced gastric cancer [1, 2]. Patients with type 4 gastric cancer have such poor survival that they are usually distinguished from other types [2, 3]. In Japan, clinical evidence for type 4 gastric cancer was established in different clinical trials [4, 5].
Total gastrectomy with splenectomy to completely dissect splenic hilar (station No. 10) lymph nodes had been the standard treatment for all types of advanced proximal gastric cancer in Japan. However, the Japan Clinical Oncology Group (JCOG) 0110 trial showed that splenectomy for No. 10 lymph node dissection should be avoided for advanced proximal gastric cancer without the invasion to the line of the greater curvature [6]. Limited numbers of Japanese patients with proximal advanced gastric cancer involving the greater curvature have undergone total gastrectomy with splenectomy since the results of this pivotal trial were published.
However, the oncological value of splenectomy for dissecting station 10 lymph nodes for proximal gastric cancer involving the greater curvature is not fully understood. Several studies used the therapeutic value index to establish the benefit of splenectomy for such disease [7,8,9]. Although the results from these studies are an excellent reference for splenectomy, the clinical question remains whether the significance of splenectomy for No. 10 lymph node dissection in the treatment of type 4 gastric cancer is the same as that of the other types, because these studies included patients with all macroscopic types of gastric cancer. Furthermore, the characteristics of type 4 gastric cancer differ from the other types mentioned above.
Type 4 gastric cancer has a poorer prognosis than that of non-type 4. Many patients with type 4 gastric cancer experience peritoneal metastasis even after curative gastrectomy [10, 11]. Furthermore, splenectomy is associated with a high incidence of severe postoperative complications [6]. Postoperative complications, such as pancreatic fistula and intra-abdominal abscess, delay, or cancel initiating postoperative adjuvant chemotherapy within the appropriate period. Moreover, severe postoperative complications affect survival outcomes in gastric cancer as well as other cancers [12,13,14]. Therefore, whether splenectomy for complete dissection of station 10 lymph nodes plays an important role in type 4 gastric cancer is not established.
In the present study, to determine whether splenectomy aimed to completely dissect station No. 10 lymph nodes is oncologically valid for type 4 gastric cancer involving the greater curvature, we retrospectively evaluated postoperative complications and the therapeutic value index of station No. 10 lymph nodes of patients with type 4 proximal gastric cancer involving the greater curvature compared with those with non-type 4 disease.
Methods
Patients
We enrolled 110 patients who underwent D2 total gastrectomy with splenectomy and achieved R0 resection for cT2-T4 gastric cancer involving the greater curvature on the upper or middle third of the stomach or both. These patients were treated at the Cancer Institute Hospital between January 2006 and December 2016 and were selected from our prospective database. The greater curvature is defined as one of four equal parts of the gastric circumference, according to the Japanese Classification of the Gastric Association (JCGA) [15]. Exclusion criteria were as follows: patients with esophageal junctional (EGJ) cancer, those with bulky lymph nodes, and those undergoing combined distal pancreatectomy, or conversion surgery. The clinical stage was classified according to the 14th Edition of the JCGA [15]. EGJ was based on the Siewert Classification [16]. The median follow-up of patients in this study was 55 months (interquartile range: 31–62 months). The Institutional Review Board of the Cancer Institute Hospital approved this study.
Surgical procedure, postoperative therapy, and follow-up
Total gastrectomy with splenectomy was performed with full mobilization of the body to the tail of the pancreas and the spleen. Lymph nodes between the splenic artery and vein were dissected along the splenic artery from the root to the distal side of the branching point of the greater pancreatic artery. The splenic artery was generally cut just distal to the greater pancreatic artery, and the fatty tissue, including the distal side of the splenic artery was removed from the pancreas. Finally, the splenic vein was cut at the pancreatic tail, and the spleen was removed en block together with stations No. 10 and 11 lymph nodes. We recently preserved the splenic artery and skeletonized as far as the splenic hilar, and the splenic branches were cut to remove the spleen. Roux-en-Y fashion reconstruction was performed following lymph node dissection. On the basis of the results of the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC) [17], adjuvant S-1 monotherapy has been performed as standard treatment in Japan since 2007. The schedule, dose, and indication of S-1 were according to the ACTS-GC protocol [17]. In our institute, adjuvant chemotherapy had been introduced as clinical practice since 2006. Therefore, this study enrolled patients who underwent surgery from 2006. Some patients who entered clinical trials were administered S-1 plus oxaliplatin or capecitabin plus oxaliplatin. In the outpatient clinic, patients were followed and evaluated for physical findings, blood tests, including the tumor markers, carcinoembryonic antigen and carbohydrate antigen 19–9, and half-annual abdominal computed tomography or ultrasonography for at least 5 years after surgery.
Evaluations
Clinicopathological outcomes, the incidence of lymph node metastasis, and 5-year overall survival (OS) at each lymph node station, and the therapeutic value index were evaluated. To evaluate the therapeutic value of dissection at each lymph node station, we used the therapeutic value index proposed by Sasako et al. [18]. The therapeutic value of nodal dissection (percentage) was obtained by multiplying the incidence of lymph node metastasis by the 5-year survival rate. The rate of lymph node metastasis was calculated by multiplying the number of patients with lymph node metastasis at each station by the number of those in whom that station was retrieved. The 5-year OS rates for patients with lymph node metastasis were calculated for each nodal station, regardless of lymph node metastasis at other stations. Differentiated types of gastric cancer included papillary and tubular adenocarcinomas. Undifferentiated types included poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma. Postoperative complications were evaluated according to the Clavien–Dindo classification (C–D) [19, 20]. Here we applied the incidence of postoperative complications and the therapeutic value index to determine whether splenectomy for type 4 gastric cancer is valid, when compared with its application to non-type 4 gastric cancer.
Statistical analysis
Fisher’s exact test and the Mann–Whitney test were used for statistical analysis. OS was estimated using the Kaplan–Meier method. All statistical analyses were performed with SPSS ver. 24.0 for Windows (IBM, Chicago, IL, USA). P < 0.05 was considered to denote statistical significance.
Results
Patients’ characteristics
Figure 1 shows a flow chart of the patients in the present study. We studied 110 patients who underwent total gastrectomy with splenectomy of tumors involving the greater curvature. These patients were divided into the type 4 group (n = 50) and the non-type 4 group (n = 60). During the study, 20 patients whose tumors invaded the greater curvature did not undergo total gastrectomy without splenectomy, and 16 patients had type 4 disease. The reasons why they did not undergo splenectomy were advanced age, generally poor preoperative condition, and a severe comorbidity such as diabetes mellitus, chronic heart failure, or renal dysfunction. The clinicopathological characteristics of patients in the type 4 and non-type 4 groups are shown in Table 1. The type 4 group had a significantly higher proportion of undifferentiated (P = 0.010) and encircling types of gastric cancer (P < 0.001) than did the non-type 4 group. The median tumor size in the type 4 group was significantly larger than that in the non-type 4 group. The incidence of clinically positive No. 10 lymph node metastasis was significantly higher in the non-type 4 group than the type 4 group (P = 0.011). In the type 4 group, more than 90% of patients had T4a disease and 60% had more than six lymph node metastases. Pathological T4b disease invading the spleen did not occur in any patient.
Postoperative complications
The overall incidence of postoperative complications was 50.0% in Grade II or higher and 20.9% in Grade IIIa or higher (Table 2). Intraabdominal abscess or infection and pancreatic fistula were frequent complications. There was no significant difference in complications between the type 4 and non-type 4 groups.
Incidence of lymph node metastasis
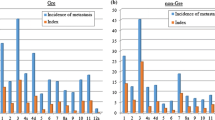
The incidence of No. 10 lymph node metastasis was 26.0% (95% confidence interval [CI] 14.6–40.3) in the type 4 group and 31.7% (95% CI, 20.3–45.0) in the non-type 4 group. These values were not significantly different. However, the incidence of stations No. 3, 4sa, 4sb, 4d, and 6 in the type 4 group was significantly higher than that in the non-type 4 group (Table 3). Therefore, station No. 10 in the type 4 group ranked ninth and that in non-type 4 group was ranked second (Fig. 4a, b).
Survival outcomes
The 5-year OS rates were 38.8% and 67.7% in the type 4 and non-type 4 groups, respectively. Patients in the type 4 group experienced significantly shorter survival than those in the non-type 4 group (P = 0.009) (Fig. 2).
Patients who experienced Grade IIIa or higher postoperative complications did not experience shorter survival than those who did not experience complications in the type 4 and non-type 4 groups (Fig. 3a, b).
Survival curves for patients with or without Grade IIIa or higher postoperative complications in the type 4 (a) and non-type 4 (b) groups. There was no significant difference in survival between patients with or without Grade IIIa or higher in both groups (P = 0.628 and P = 0.276, respectively). Survival curves for patients with and without No. 10 lymph node metastasis in the type 4 (c) and non-type 4 (d) groups. There was no significant difference in survival between patients with and without No. 10 lymph node metastasis in the type 4 group (P = 0.638). However, the survival of patients with No. 10 lymph node metastasis was significantly shorter than that of patients without No. 10 lymph node metastasis in the non-type 4 group (P = 0.008)
Patients with or without No. 10 lymph node metastasis in the type 4 group had the same survival outcomes. Patients with No. 10 lymph node metastasis had a significantly poorer survival outcome than patients without No. 10 lymph node metastasis in the non-type 4 group (P = 0.008) (Fig. 3c, d).
Therapeutic value index of each lymph node station
The 5-year OS rates of patients with No. 10 lymph node metastasis in the type 4 and non-type 4 groups were 52.7% and 47.4%, respectively. Therefore, the therapeutic value index for station No. 10 was 13.7 in the type 4 group and 15.0 in the non-type 4 group (Table 3; Fig. 4c, d). Although the indices of station No. 10 were similar in the type 4 and non-type 4 groups, the rank of this index of the type 4 group was seventh and that in the non-type 4 group was second. The therapeutic value index of station No. 10 in the type 4 group ranked just below those of peri-gastric stations Nos. 2, 3, 4sa, and 4d. Moreover, the index of station No. 10 was comparable to that of station No. 7 and higher than the indices of the other suprapancreatic stations (Nos. 8a, 9, 11p, 11d, 12a). However, the index of station No. 10 in the non-type 4 group was lower than that of only station No. 3 was equal to that of station No. 4d and was higher than those of the other suprapancreatic stations.
The incidence of lymph node metastasis and the therapeutic index for each station in descending order in the type 4 and non-type 4 groups. a Incidence of lymph node metastasis in the type 4 group. The incidence of No. 10 lymph node metastasis was ranked as ninth and was lower than that in the peri-gastric area. b Incidence of lymph node metastasis in the non-type 4 group. The incidence of No. 10 lymph node metastasis was ranked as second and was higher than indices of the peri-gastric area, except for station No. 3. c Therapeutic value index of the type 4 group. The therapeutic value index of station No. 10 was equal to that of No. 7 and higher than indices of the other supra-pancreatic areas. d Therapeutic value index of the non-type 4 group. The incidence of station No. 10 ranked second and equal to No. 4d
Table 4 shows the therapeutic value indices of station No. 10, which were calculated according to the clinical status of No. 10 lymph node metastasis. Only one patient in the type 4 group had clinical metastasis, although the swollen lymph node did not harbor detectable metastasis. Thus, the therapeutic value indices of patients with or without clinical metastasis were 0 and 14, respectively. In the non-type 4 group, the therapeutic value index was 40 for patients with clinical metastasis, which was higher than 10 for those without clinical metastasis.
Discussion
In the present study, three key information regarding splenectomy to completely dissect station No.10 lymph nodes for patients with type 4 gastric cancer involving the greater curvature was obtained. First, the incidence of complications after total gastrectomy with splenectomy for the type 4 group was high, but not different from the non-type 4 group. Second, the therapeutic value index of station No. 10 in the non-type 4 group was high and ranked just below that of station No. 3. Third, the therapeutic value index of station No. 10 in the type 4 group was comparable to that in the non-type 4 group. It was also comparable to station No. 7 and higher than those of the other suprapancreatic stations. These findings may indicate that splenectomy to dissect station No.10 lymph nodes is oncologically valid for type 4 gastric cancer involving the greater curvature as well as non-type 4 disease.
Several reports show that the incidence of No. 10 lymph node metastasis in type 4 gastric cancer is 26.4–34.1% [21,22,23]. Although these studies did not consider whether the tumor involved the greater curvature, the incidence of No. 10 lymph node metastasis (26.0%) in the present study is comparable. This is explained by findings that 75.6–81.2% of type 4 gastric cancer involves the greater curvature [7, 8]. In the preset study, the incidence of No. 10 lymph node metastasis was not significantly different between the type 4 and non-type 4 groups, although the ranks of the groups and the influence of survival outcomes differed. Type 4 gastric cancer is longer and usually larger in circumference compared with non-type 4 gastric cancer, while non-type 4 gastric cancer involving the greater curvature is localized to a limited area. These findings may explain why the rank of the incidence of No. 10 lymph node metastasis was seventh in the type 4 group, while it was second in the non-type 4 group. Furthermore, No. 10 lymph node metastasis did not affect survival outcomes in the type 4 group, while No. 10 lymph node metastasis significantly shortened them in the non-type 4 group. We were unable to account for the difference; but possible reasons may be that splenectomy prolonged the survival rates of patients with No. 10 lymph node metastasis, or peritoneal metastasis more influenced survival outcomes than No. 10 lymph node metastasis in the type 4 group.
We calculated the therapeutic value index by multiplying the metastatic incidence by the 5-year OS to assess the benefit of lymph node dissection at each station. The therapeutic value indices in the type 4 group were higher, particularly in all peri-gastric stations, than those in the non-type 4 group. This finding suggests that patients with peri-gastric lymph node metastasis in the type 4 group benefitted from dissection, although the incidence of peri-gastric lymph node metastasis in the type 4 group was higher than that in the non-type 4 group at each station. Furthermore, the therapeutic value index of station No. 10 in the type 4 group was comparable to that of station No. 7 and higher than that in the other supra-pancreatic areas, Nos. 8a, 9, and 11d. These findings indicate that No. 10 lymph node dissection for type 4 gastric cancer involving the greater curvature should be performed, as well as lymph node dissection in the supra-pancreatic area that is usually subject to D2 dissection. In the non-type 4 group, the therapeutic value index of station No. 10 was lower than that of only station No. 3 and equivalent to that of station No. 4d. Furthermore, the index of station No. 10 was higher than that in the other peri-gastric and supra-pancreatic stations. Station No. 10 lymph nodes represent main stations that undergo dissection in non-type 4 gastric cancer involving the greater curvature.
The therapeutic value indices of the type 4 and non-type 4 groups in the current study were comparable and remarkably higher than those in previous studies [7,8,9]. Some possible reasons for this difference are as follows: First, patients were strictly selected in the present study. Other studies included patients with EGJ cancer, those with bulky nodal metastasis, or those who underwent combined distal pancreatectomy [7,8,9]. These characteristics; in particular, are associated with poor survival. In the present study, exclusion of such patients may have resulted in a better therapeutic value index. Another reason for a higher index in our study may be that longer survival was achieved through the administration of adjuvant chemotherapy.
The 5-year OS in our study was equal to that of previous studies [7,8,9, 21], although our study included patients with a more advanced pN category. Other studies were conducted before 2007, when the standard adjuvant chemotherapy for pathological Stage II or III gastric cancer was not yet established in Japan [17]. Therefore, only 20.2–49.0% of patients in these studies underwent adjuvant chemotherapy [7,8,9, 21], while 81.8% of patients received such chemotherapy in our study. This high proportion of those who were administered adjuvant chemotherapy led to longer 5-year OS by stage compared with previous studies. The longer survival outcomes achieved better therapeutic value indices of each station in the present study, which may be explained by the high therapeutic value index of type 4 gastric cancer. Furthermore, our data for patients treated according to the current therapeutic strategy are more applicable to daily practice than those previously reported [7,8,9, 21].
Splenectomy for No. 10 lymph node dissection presents a crucial problem. In the JCOG0110 trial, Sano et al. [6] reported that the incidences of complications associated with total gastrectomy with splenectomy and without splenectomy were 30.3% and 16.7%, respectively (P < 0.01) [6], and that pancreatic fistula or abdominal abscess occurred more frequently in patients who underwent splenectomy than those who did not. We believe that pancreatic fistula may be caused by touching or pushing the pancreas by surgeons, the application of heat generated by electrocautery, and circulatory failure when an artery or vein is incised. For example, we reported that touching the pancreas induces leakage of pancreatic juice [26]. During mobilization of the pancreas and dissection of the lymph nodes around the splenic artery and vein, the pancreas can be touched or pushed by surgeons’ hands. Such blunt trauma may be a major factor that causes pancreatic fistula. According to such recognition, we have recently dissected station No.11 lymph nodes with gentle or non-touch maneuver for the pancreas besides preservation of the splenic artery to the pancreatic tail for maintaining the caudal circulation. These procedures may decrease occurrence of pancreatic fistula.
Dutch Gastric Cancer Trial revealed that splenectomy was the most important risk factor for overall complications, and the patients undergoing splenectomy with D1 and D2 lymphadenectomy experienced significantly lower overall survival [24, 25]. Others found that postoperative abdominal infectious complications were independent risk factors for poor compliance with adjuvant S-1 chemotherapy, and delayed administration after 8 weeks was associated with a greater risk of recurrence [27, 28]. These reports show that the occurrence of severe postoperative complications is an independent prognostic factor. Therefore, splenectomy in total gastrectomy may have two opposing effects: curing disease and preventing a cure. Although patients who experience postoperative complications did not experience worse survival outcomes here, surgeons should strive to decrease such complications to achieve longer survival. For example, Oh et al. [29] reported that although spleen-preserving lymphadenectomy in patients with proximal gastric cancer did not affect long-term outcomes (recurrence and OS), it achieved better short-term surgical outcomes than splenectomy for lymph node dissection [29]. Spleen-preserving No. 10 lymphadenectomy may therefore be useful for decreasing complications without affecting the efficiency of No. 10 lymph node dissection.
This study has several limitations. First, this retrospective study analyzed patients treated at a single institution, did not compare splenectomy with no splenectomy, and evaluated a relatively small number of patients (n = 110). However, such preliminary results may provide the foundation for future confirmatory studies. Second, there was selection bias. Although the standard procedure for advanced proximal gastric cancer was total gastrectomy with splenectomy here, splenectomy was not administered to older patients, as well to those with low performance status or a severe comorbidity. Among patients who did not undergo splenectomy, even though the tumor invaded the greater curvature, four fifth of them had type 4 disease. Therefore, our patients’ backgrounds in both groups were not balanced. Third, the therapeutic index is theoretical. This index does not consider patients’ characteristics, tumor factors, surgical factors, and perioperative therapy. Thus, the results of our study are not definitive. Because of the high incidence of postoperative complications, the oncological feasibility of spleen-preserving No. 10 lymph node dissection requires further evaluation to establish its enhanced safety.
In conclusion, splenectomy for No. 10 lymph node dissection may be oncologically valid for patients with type 4 gastric cancer involving the greater curvature. However, there was a high incidence of postoperative complications associated with total gastrectomy with splenectomy. Safer No. 10 lymph node dissection should be achieved to decrease complications after surgery for proximal advanced gastric cancer involving the greater curvature, regardless of gross tumor types.
References
Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer. 1998;1:125–33.
An JY, Kang TH, Choi MG, Noh JH, Sohn TS, Kim S. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J Gastrointest Surg. 2008;12:1364–9.
Otsuji E, Kuriu Y, Okamoto K, Ochiai T, Ichikawa D, Hagiwara A, et al. Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg. 2004;188(3):327–32.
Kinoshita T, Sasako M, Sano T, Katai H, Furukawa H, Tsuburaya A, et al. Phase II trial of S-1 for neoadjuvant chemotherapy against scirrhous gastric cancer (JCOG 0002). Gastric Cancer. 2009;12:37–42.
Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, et al. Gastric Cancer Surgical Study Group of Japan Clinical Oncology Group. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107:741–5.
Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al. (2017) Stomach Cancer Study Group of the Japan Clinical Oncology Group. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg. 2017;265:277–83.
Yura M, Yoshikawa T, Otsuki S, Yamagata Y, Morita S, Katai H, et al. The Therapeutic Survival Benefit of Splenic Hilar Nodal Dissection for Advanced Proximal Gastric Cancer Invading the Greater Curvature. Ann Surg Oncol. 2019;26:829–35.
Watanabe M, Kinoshita T, Enomoto N, Shibasaki H, Nishida T. Clinical Significance of Splenic Hilar Dissection with Splenectomy in Advanced Proximal Gastric Cancer: An Analysis at a Single Institution in Japan. World J Surg. 2016;40:1165–71.
Maezawa Y, Aoyama T, Yamada T, Kano K, Hayashi T, Sato T, et al. Priority of lymph node dissection for proximal gastric cancer invading the greater curvature. Gastric Cancer. 2018;21:569–72.
Moriguchi S, Maehara Y, Korenaga D, Sugimachi K, Nose Y. Risk factors which predict pattern of recurrence after curative surgery for patients with advanced gastric cancer. Surg Oncol. 1992;1:341–6.
Kitamura K, Beppu R, Anai H, Ikejiri K, Yakabe S, Sugimachi K, Saku M. Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol. 1995;58:112–7.
Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol. 2014;21:891–8.
Lerut T, Moons J, Coosemans W, Van Raemdonck D, De Leyn P, Decaluwé H, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg. 2009;250:798–807.
Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240:255–9.
Japanese classification of gastric carcinoma: 3rd English edition. Japanese Gastric Cancer Association Gastric Cancer 2011;14:101–12
Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–9.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346–51.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg. 2004;240:205–13.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Kosuga T, Ichikawa D, Okamoto K, Komatsu S, Shiozaki A, Fujiwara H, et al. Survival benefits from No 10 lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer. 2011;14:172–7.
Shin SH, Jung H, Choi SH, An JY, Choi MG, Noh JH, et al. Clinical significance of splenic hilar lymph node metastasis in proximal gastric cancer. Ann Surg Oncol. 2009;16:1304–9.
Jeong O, Jung MR, Ryu SY. Clinicopathological features and prognostic impact of No. 10 lymph node metastasis in proximal gastric carcinoma. Eur J Surg Oncol. 2019;45:432–8.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49.
Sasako M. Risk factors for surgical treatment in the Dutch Gastric Cancer Trial. Br J Surg. 1997;84:1567–71.
Ida S, Hiki N, Ishizawa T, Kuriki Y, Kamiya M, Urano Y, et al. Pancreatic Compression during Lymph Node Dissection in Laparoscopic Gastrectomy: Possible Cause of Pancreatic Leakage. J Gastric Cancer. 2018;18:134–41.
Nakanishi K, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. Delay in initiation of postoperative adjuvant chemotherapy with S-1 monotherapy and prognosis for gastric cancer patients: analysis of a multi-institutional dataset. Gastric Cancer. 2019. https://doi.org/10.1007/s10120-019-00961-9.
Yamashita K, Kurokawa Y, Yamamoto K, Hirota M, Kawabata R, Mikami J, et al. Risk Factors for Poor Compliance with Adjuvant S-1 Chemotherapy for Gastric Cancer: A Multicenter Retrospective Study. Ann Surg Oncol. 2017;24:2639–45.
Oh SJ, Hyung WJ, Li C, Song J, Kang W, Rha SY, et al. The effect of spleen-preserving lymphadenectomy on surgical outcomes of locally advanced proximal gastric cancer. J Surg Oncol. 2009;99:275–80.
Acknowledgements
Yosuke Kano and Manabu Ohashi contributed equally to this study. The authors declare that no external funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the institutional and national committees governing human experimentation and in compliance with the Helsinki Declaration of 1964 and later versions. Informed consent or an appropriate substitute was obtained from all patients prior to their inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kano, Y., Ohashi, M., Ida, S. et al. Therapeutic value of splenectomy to dissect splenic hilar lymph nodes for type 4 gastric cancer involving the greater curvature, compared with other types. Gastric Cancer 23, 927–936 (2020). https://doi.org/10.1007/s10120-020-01072-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01072-6