Abstract
Background
Treatment of patients with advanced or metastatic esophagogastric adenocarcinoma should not only prolong life but also provide relief of symptoms and improve quality of life (QOL). Esophagogastric adenocarcinoma mainly occurs in elderly patients, but they are underrepresented in most clinical trials and often do not receive effective combination chemotherapy, most probably for fear of intolerance. Using validated instruments, we prospectively assessed QOL within the randomized FLOT65+ phase II trial.
Methods
Within the FLOT65+ trial, a total of 143 patients aged ≥65 years were randomly allocated to receive biweekly oxaliplatin plus 5-fluorouracil (5-FU) continuous infusion and folinic acid (FLO) or the same regimen in combination with docetaxel 50 mg/m2 (FLOT). The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) and the gastric module STO22 were administered every 8 weeks until progression. Time to definitive deterioration of QOL parameters was analyzed and compared within the treatment arms.
Results
The median age of patients was 70 years. Patients receiving FLOT exhibited higher response rates and had improved disease-free and progression-free survival (PFS). The proportions of patients with evaluable baseline EORTC QLQ-C30 and STO22 questionnaires were balanced (83 % in FLOT and 89 % in FLO). Considering evaluable patients with assessable questionnaires (n = 123), neither functioning nor symptom parameters differed significantly in favor of one of the two treatment groups. Particularly, there was no significant difference regarding time to definitive deterioration of global health status/quality of life from baseline (primary endpoint). Notably, patients receiving FLO or FLOT as palliative treatment (n = 98) achieved comparable QOL results.
Conclusions
Although toxicity was higher in patients receiving FLOT, no negative impact of the addition of docetaxel on QOL parameters could be demonstrated. Thus, elderly patients in need of intensified chemotherapy may receive FLOT without compromising patient-reported outcome parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gastric cancer is the second most common cause of cancer death worldwide [1]. Overall survival is poor, with the majority of patients presenting with advanced, unresectable disease [2]. Current treatment may barely improve survival but only tumor-related symptoms in these patients. As a result, quality of life (QOL) is particularly important for patients with incurable disease [3]. The TAX 325 phase III trial represents the largest trial with the longest prospectively controlled evaluations of QOL during protocol chemotherapy and follow-up in patients with advanced esophagogastric adenocarcinoma: 445 patients randomly received either docetaxel and cisplatin each on day 1 plus 5-FU continuous infusion on day 1–5 every 3 weeks (DCF) or cisplatin on day 1 plus 5-FU continuous infusion on days 1–5 every 4 weeks (CF). QOL was assessed administering the EORTC QLQ-C30 and the EuroQOL EQ-5D questionnaire every 8 weeks from baseline until progression. Patients receiving DCF not only had statistically improved overall survival and time to tumor progression, but they also exhibited better preservation of QOL compared with patients receiving CF [4]. Esophagogastric adenocarcinoma is mainly diagnosed in elderly patients. Nevertheless, the elderly are generally underrepresented in most clinical trials and often do not receive effective combination chemotherapy, most probably for fear of intolerance. In TAX 325, for instance, only 24 % of the patients receiving docetaxel were aged 65 years or older [5].
Based on results of a preceding phase II trial [6], the aim of the randomized FLOT65+ phase II trial was to establish FLOT as an alternative therapy concept to DCF in elderly patients. The study evaluated the efficacy and safety of docetaxel, 5-FU, and oxaliplatin (FLOT) compared with oxaliplatin and 5-FU (FLO) in 143 patients with advanced or metastatic gastric cancer who were aged 65 or older. The efficacy results favored FLOT over FLO, with FLOT resulting in higher response rate (49 vs. 28 %; χ2 test, P = 0.010) and a longer PFS (P = 0.048) [7]. There was no statistically significant difference in median overall survival between the two groups. Grade 3 or 4 toxicities occurred in 82 % of patients in the FLOT arm and in 39 % of patients in the FLO arm (P < 0.001). Higher rates of grade 3 and 4 neutropenia, diarrhea, and nausea were associated with FLOT, but there was no increase in severe adverse events, interruption of treatment, or deaths caused by therapy [7].
In the present analysis we sought to investigate if FLOT, representing an effective treatment with a high response rate and an acceptable toxicity profile compared to FLO, as an established treatment option in elderly patients may improve QOL by palliating or stabilizing symptoms or if the benefit is counterbalanced by the burden of treatment-associated toxicity.
Methods
The design of FLOT65+ is described elsewhere [7]. Briefly, this randomized phase II trial design is summarized in the following sections.
Inclusion and exclusion criteria
Chemotherapy-naive patients aged ≥65 years with metastatic, locally advanced, or recurrent esophagogastric adenocarcinoma, ECOG performance status ≤2, and adequate hematological, renal, and hepatic functions were enrolled. Main exclusion criteria were as follows: intolerance to 5-FU, folinic acid, oxaliplatin, or docetaxel; active coronary heart disease or heart failure (stages II–IV according to the New York Heart Association); serious internal or acute infection disease; peripheral neuropathy more than grade 2; known brain metastases; liver impairment with ALT and/or AST more than 3.5× the upper limit of normal, alkaline phosphatase more than 6× the upper limit of normal, and bilirubin more than 1.5× the upper limit of normal; inflammatory bowel disease; participation in another study. Written informed consent was obtained from all patients.
Treatment
Patients were randomly assigned to receive either oxaliplatin 85 mg/m2 plus 5-FU 2,600 mg/m2 continuous infusion and folinic acid 200 mg/m2 each on day 1 every 2 weeks alone (FLO) or in combination with docetaxel 50 mg/m2 (FLOT). Randomization was stratified for center, stage of disease (metastatic versus locally advanced), presence or absence of liver metastases, ECOG performance status (0 and 1 versus 2), and pharmacological profile (high versus low risk). Potentially resectable patients received four cycles of chemotherapy before and after resection if peritoneal carcinomatosis was excluded by laparoscopy before beginning of treatment.
QOL: patient questionnaires
Quality of life was assessed using two self-administered questionnaires: the generic European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30), version 3.0, and the disease-specific module STO22. The EORTC QLQ-C30 questionnaire is a validated, cancer-specific instrument designed for prospective clinical trials [8]. The questionnaire incorporates five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, and nausea/vomiting), a global health status (GHS)/QOL scale, and six single items assessing additional symptoms commonly reported by cancer patients (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). The STO22 is a gastric cancer module to be administered in addition to the core questionnaire. It includes 22 questions, conceptualized as consisting of five scales (dysphagia, pain, reflux symptoms, eating restrictions, and anxiety) and four single items (having a dry mouth, taste, body image, and hair loss), related to disease symptoms, treatment side effects, nutritional aspects, and emotional issues specific to gastric cancer [9]. It is also validated and designed for use in cancer clinical trials [10].
QOL was assessed before random assignment, every 8 weeks during chemotherapy at the same time as tumor assessment but before informing the patient about the disease evolution, and before chemotherapy infusion, every 8 weeks during the follow-up period up to 6 months after the end of treatment.
QOL was compared between the two arms over the entire study period (during chemotherapy and follow-up). The baseline QOL questionnaire had to be completed ≤7 days before random assignment and ≤14 days before start of treatment to be considered assessable; the assessments during the treatment period were planned every 8 weeks before reevaluation by computer tomography (CT) and before chemotherapy infusion. Follow-up implied a time of 6 months after the end of treatment and included reevaluation by CT to document progression and QOL. Questionnaires without an assessment date were not assessable.
Statistical methods
Following the EORTC guidelines [11], the 30 items of the QLQ-C30 were transformed into the 15 scales mentioned earlier. A linear transformation was used to obtain scales ranging from 0 to 100, with a high score indicating a high level of functioning for the functional and global scales and, conversely, a high level of symptomatology for the symptoms scales. Missing items within scale were handled according to the EORTC recommendations [11].
The 22 items of the STO22 scales were transformed into 5 scales and 4 single items as already mentioned. The transformation procedure is equivalent. The scoring algorithms for the scales have been described in a similar fashion to the scoring for the EORTC QLQ-C30. For the symptom scales and items, a high score is equivalent to worse or more symptoms. In the functional scales, however, a high score is equivalent to better function.
The primary endpoint of the QOL assessment was time to definitive deterioration from baseline by 5 % on the GHS/QOL scale. Secondary endpoints were time to definitive deterioration of GHS/QOL from baseline by 10, 15, and 20 % and time to definitive deterioration of all other scales of the QLQ-C30 and the STO22 by 5, 10, 15, and 20 % (for each parameter).
Definitive deterioration was calculated by the chi-square test as relative deviation from baseline. If data were too few, deterioration was evaluated by the Fisher’s exact test.
Subgroup analyses were performed in patients with inoperable or metastatic disease. Differences in GHS/QOL of patients <70 and ≥70 years (regardless of treatment arm) were investigated by exploratory analyses.
Results
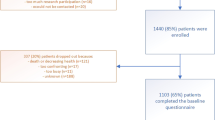
A total of 143 patients from 28 centers in Germany were enrolled and treated (FLOT, n = 72; FLO, n = 71). General baseline characteristics of the patients groups were comparable. Median age was 70 years (Table 1).
The proportion of patients with assessable QLQ-C30 questionnaire at baseline was 83 % with FLOT and 89 % with FLO (Table 2). The rate of assessable questionnaires over time is depicted in Table 2. A comparable percentage of questionnaires were available for the two treatment groups.
There were no differences between the groups at baseline in the QLQ-C30 GHS/QOL or symptom subscales, but physical functioning and social functioning were significantly better in the FLO group (Table 3).
There were no differences between the groups at baseline in the STO22 functional or symptom subscales, except anxiety, which was higher in the FLOT group. Eating restrictions were common in both groups (Table 3).
Regarding primary endpoint and considering all patients for all thresholds (5, 10, 15, and 20 %), time to definitive deterioration of GHS/QOL from baseline occurred slightly later with FLO than with FLOT, but this was not significant for any time point (P = 0.270–0.639) (Table 4).
A clinically relevant moderate to large (≥10 points) deterioration of QOL GHS scores during the first 8 weeks of treatment occurred in 19 of 40 (47.5 %) patients with FLOT compared to 9 of 44 (20.5 %) patients with FLO who were evaluable for QOL GHS scores (P = 0.011; data not shown).
For the other functioning or symptom parameters, all analyses (deterioration by 5, 10, 15, and 20 %) did not significantly favor one of the two treatment groups (data not shown). The only exception was the physical functioning parameter, for whom time to deterioration by 5 and 20 % occurred significantly later with FLO than FLOT (P = 0.030 and 0.047, respectively). Nevertheless, deterioration by 10 and 15 % was not significantly different between the two treatment arms (P = 0.076 and 0.092, respectively).
Regarding the subgroup of patients treated with palliative intent, the analysis of the primary endpoint (time to 5 % definitive deterioration of GHS/QOL) delivered virtually identical results with FLO and with FLOT, respectively (P = 0.772) (Fig. 1). Accordingly, all other thresholds (10, 15, and 20 % deterioration of GHS) did not differ between the two treatment groups in palliative patients (data not shown). Regarding the functioning and symptom parameters, there was also no difference between the two treatment arms for any threshold (data not shown).
Exploratory analyses regardless of treatment arm were performed in patients <70 and ≥70 years. Results showed no differences in time to 5 % definitive deterioration of GHS/QOL between the two groups (P = 0.666) (Fig. 2). All other thresholds (10, 15, and 20 % deterioration of GHS) did not differ between patients <70 and ≥70 years. Regarding the functioning and symptom parameters, differences were seen in time to 20 % definitive deterioration of social functioning (<70 years, 127 days; ≥70 years, 139 days; P = 0.047) and in time to 10 % definitive deterioration of anxiety, which occurred after 120 days in patients <70 years and 60 days in patients ≥70 years (P = 0.030).
Conclusions
Chemotherapy can maintain QOL in patients with advanced esophagogastric adenocarcinoma [12]. To date, there have been few trials focusing on elderly patients aged 65 years or older, and no randomized trials in this patient population had been reported. The aim of the multicenter, randomized, phase II trial FLOT65+ was to establish FLOT as an alternative treatment in elderly patients.
The addition of docetaxel to 5-FU/folinic acid and oxaliplatin resulted in significant improvements in response rate and PFS, although there was no statistically significant difference in median overall survival between the two groups [7].
In a prespecified subgroup analysis, the increase in efficacy with FLOT was more evident in patients with resectable disease, resulting in a statistically significant improvement in overall response rate (62 vs. 19 %) and median PFS compared to FLO (24.2 vs. 10.3 months), whereas the benefit for patients with unresectable or metastatic disease was smaller, suggesting the most beneficial role of treatment intensification in patients with potentially resectable disease [7].
The type and percentage of distinct adverse events observed within the FLOT arm of FLOT65+ were consistent with what had previously been reported for FLOT in younger patient collectives [6]. However, compared with FLO, a significant increase in overall grade 3 and 4 toxicity was seen (FLOT, 82 %; FLO, 39 %; P < 0.001), especially considering neutropenia, leukopenia, diarrhea, and nausea [7].
The main question investigated in the present analysis was if FLOT representing an effective treatment with a high response rate and an acceptable toxicity profile improved QOL of elderly patients compared to FLO.
The QOL results presented here rely on a total of only 143 eligible patients, and therefore the results should be interpreted with caution. Nevertheless, the collective of elderly patients is unique, and the present analysis is the first to report on QOL data from a prospective trial in elderly. Moreover, a high percentage of questionnaires could be collected throughout the entire study period.
In the present trial, the median age of patients was 70 years. Mean score of GHS at baseline was 49 in the FLOT group and 56 in the FLO group. The reference data in the German population indicates a better quality of life, with a mean score of 61.5 for men and 78.9 for women ≥70 years [13]. There were no statistically significant differences between the two treatment arms concerning most of the evaluated quality of life parameters at baseline. An exception was the physical functioning parameter, which was significantly better in the FLO group. Regarding the evolution of quality of life parameters during the study there was no difference between the treatment groups besides physical functioning, for whom time to definitive deterioration by 5 and 20 % occurred significantly later with FLO than FLOT. Thus, a better physical functioning at baseline in the FLO group may have been protective from early deterioration compared with the FLOT group.
Time to 5 % definitive deterioration of GHS from baseline was the primary endpoint. When FLOT65+ was initiated data from Osoba et al. [14] indicated that deterioration of GHS by ≥10 points corresponds to a clinically relevant moderate deterioration of quality of life, as it was seen during the first 8 weeks of treatment in this study. On the other hand, FLOT65+ investigated the addition of docetaxel to a platinum- and 5-FU based chemotherapy doublet. In this regard, FLOT65+ was comparable to the study published by Ajani et al. [4]. In the TAX 325 trial, however, the primary endpoint of the QoL analysis was deterioration of the GHS/QoL scale by 5 %. To be comparable with these data, we decided to investigate a 5 % deterioration as well. Meanwhile, with another publication by Cocks and coworkers [15] strong arguments are at hand that the deterioration of 10 points might serve as a reasonable endpoint in such analyses. In view of the publications of Osoba et al. and Cocks et al., we believe that for further research in this field a time to definitive deterioration by 10 points is a reasonable endpoint.
The primary endpoint in the present study occurred after 73 days with FLOT and 119 days with FLO without reaching statistical significance. The increase in efficacy with FLOT did not result in a better QOL regarding the whole patient group. In contrast to the TAX 325 trial, where the addition of docetaxel resulted in a significantly longer preservation of QOL despite a higher incidence of toxicities [4], the median age of patients was considerably higher in the FLOT65+ trial than in the TAX 325 trial. In this study only 24 % of the patients receiving docetaxel were aged 65 years or older [4]. Moreover, the median performance status was worse in the FLOT65+ trial where patients presented with ECOG 1 (≈Karnofsky status of 70 and 80 %) whereas in the TAX 325 trial median Karnofsky score was 90 % [4].
Because the results from the present trial suggested a more beneficial role of chemotherapy intensification with docetaxel concerning efficacy in patients with potentially operable disease, we investigated QOL of patients with inoperable or metastatic disease in a subgroup analysis. There was no indication that elderly patients with metastatic disease may experience a negative impact on their QOL when treated with FLOT compared to FLO. Time to 5 % deterioration of GHS occurred concurrently compared to the entire patient group.
In summary, patients in need of tumor remission may receive FLOT according to the results of this analysis. Even though toxicity was higher with FLOT, the addition of docetaxel had no negative impact on QOL. In particular, elderly patients in need of tumor remission (for instance for reasons of tumor obstruction) or with locally advanced potentially resectable disease, when downsizing is a treatment goal, FLOT may represent a valid therapeutic option.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Jiang Y, Kimchi ET, Montero AJ, Staveley-O’Carroll KF, Ajani JA. Upper gastrointestinal tumors: current status and future perspectives. Expert Rev Anticancer Ther. 2008;8(6):975–91.
Aaronson NK, Bullinger M, Ahmedzai S. A modular approach to quality-of-life assessment in cancer clinical trials. Recent Results Cancer Res. 1988;111:231–49.
Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25(22):3210–6.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–7.
Al-Batran SE, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, Probst S, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19(11):1882–7.
Al-Batran SE, Pauligk C, Homann N, Hartmann JT, Moehler M, Probst S, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur J Cancer 2012 (in press).
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A; on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual. 3rd edn. European Organisation for Research and Treatment of Cancer, Brussels; 2001.
Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37(8):966–71.
Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40(15):2260–8.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Al-Batran SE, Ajani JA. Impact of chemotherapy on quality of life in patients with metastatic esophagogastric cancer. Cancer (Phila). 2010;116(11):2511–8.
Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37(11):1345–51.
Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–44.
Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 2011;29(1):89–96.
Conflict of interest
The authors indicate no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kripp, M., Al-Batran, SE., Rosowski, J. et al. Quality of life of older adult patients receiving docetaxel-based chemotherapy triplets for esophagogastric adenocarcinoma: a randomized study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Gastric Cancer 17, 181–187 (2014). https://doi.org/10.1007/s10120-013-0242-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-013-0242-1