Abstract
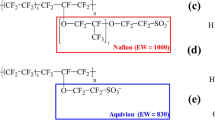
The perfluorosulfonic acid (PFSA) membrane doped with two-dimensional conductive filler Ti3C2Tx is a fuel cell proton exchange membrane with high application potential. Experimental studies showed that the proton conductivity of Nafion/Ti3C2Tx composite membrane is improved significantly compared with that in pure Nafion. However, the microscopic mechanism of doping on the enhancement of membrane performance is remain unclear now. In this work, molecular dynamics simulation was used to investigate the microscopic morphology and proton transport behaviors of Nafion/Ti3C2Tx composite membrane at the molecular level. The results shown that there were significant differences about the diffusion kinetics of water molecules and hydroxium ions in Nafion/Ti3C2Tx at low and high hydration levels in the nanoscale region. With the increase of water content, Ti3C2Tx in membrane was gradually surrounded by ambient water molecules to form a hydration layer, and forming a relatively continuous proton transport channel between Nafion polymer and Ti3C2Tx monomer. The continuous proton transport channel could increase the number of binding sites of proton and thus achieving high proton conductivity and high mobility of water molecules at higher hydration level. The current work can provide a theoretical guidance for designing new type of Nafion composite membranes.
Similar content being viewed by others
References
Xu, T. C.; Wang, C. S.; Hu, Z. Y.; Zheng, J. J.; Jiang, S. H.; He, S. J.; Hou, H. Q. High strength and stable proton exchange membrane based on perfluorosulfonic acid/polybenzimidazole. Chinese J. Polym. Sci. 2022, 40, 764–771.
Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; Huo, S.; Brandon, N. P.; Yin, Y.; Guiver, M. D. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369.
Tellez Cruz, M. M.; Escorihuela, J.; Solorza Feria, O.; Compan, V. Proton exchange membrane fuel cells (PEMFCs): advances and challenges. Polymers 2021, 13, 3046.
Hua, Z.; Zheng, Z.; Pahon, E.; Péra, M. C.; Gao, F. A review on lifetime prediction of proton exchange membrane fuel cells system. J. Power Sources 2022, 529, 231256.
Chen, X.; Xiao, L.; Qiu, X. S.; Chen, K. C. Properties of multiblock sulfonated poly(arylene ether sulfone)s synthesized by precise controllable post-sulfonation for proton exchange membranes. Chinese J. Polym. Sci. 2022, 40, 754–763.
Kusoglu, A.; Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104.
Okonkwo, P. C.; Ben Belgacem, I.; Emori, W.; Uzoma, P. C. Nafion degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: a review. Int. J. Hydrogen Energy 2021, 46, 27956–27973.
Pan, M.; Pan, C.; Li, C.; Zhao, J. A review of membranes in proton exchange membrane fuel cells: transport phenomena, performance and durability. Renew. Sust. Energy Rev. 2021, 141, 110771.
Hickner, M. A.; Ghassemi, H.; Kim, Y. S.; Einsla, B. R.; McGrath, J. E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612.
Yang, C. A comparison of physical properties and fuel cell performance of Nafion and zirconium phosphate/Nafion composite membranes. J. Membr. Sci. 2004, 237, 145–161.
He, H.; Zhu, Y.; Li, T.; Song, S.; Zhai, L.; Li, X.; Wu, L.; Li, H. Supramolecular anchoring of polyoxometalate amphiphiles into Nafion nanophases for enhanced proton conduction. ACS Nano 2022, 16, 19240–19252.
Liu, Q.; Li, Z.; Wang, D.; Li, Z.; Peng, X.; Liu, C.; Zheng, P. Metal organic frameworks modified proton exchange membranes for fuel cells. Front. Chem. 2020, 8, 694.
Beauger, C.; Lainé, G.; Burr, A.; Taguet, A.; Otazaghine, B. Improvement of Nafion®-sepiolite composite membranes for PEMFC with sulfo-fluorinated sepiolite. J. Membr. Sci. 2015, 495, 392–403.
Tsai, C. H.; Wang, C. C.; Chang, C. Y.; Lin, C. H.; Chen Yang, Y. W. Enhancing performance of Nafion®-based PEMFC by 1-D channel metal-organic frameworks as PEM filler. Int. J. Hydrogen Energy 2014, 39, 15696–15705.
Liu, X.; Yuan, H.; Wang, C.; Zhang, S.; Zhang, L.; Liu, X.; Liu, F.; Zhu, X.; Rohani, S.; Ching, C.; Lu, J. A novel PVDF/PFSA-g-GO ultrafiltration membrane with enhanced permeation and antifouling performances. Sep. Purif. Technol. 2020, 233, 116038.
Wang, C.; Zhang, L.; Yuan, H.; Fu, Y.; Zeng, Z.; Lu, J. Preparation of a PES/PFSA-g-MWCNT ultrafiltration membrane with improved permeation and antifouling properties. New J. Chem. 2021, 45, 4950–4962.
Hao, J.; Li, X.; Yu, S.; Jiang, Y.; Luo, J.; Shao, Z.; Yi, B. Development of proton-conducting membrane based on incorporating a proton conductor 1,2,4-triazolium methanesulfonate into the Nafion membrane. J. Energy Chem. 2015, 24, 199–206.
Lu, Y. H.; Cao, Y.; Lu, Y. W.; Yang, T. Thermal stability and lifetime of [AMIM]Cl-PFSA composite membranes. J. Therm. Anal. Calorim. 2017, 128, 1601–1615.
Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Enhancement in proton conductivity and thermal stability in Nafion membranes induced by incorporation of sulfonated carbon nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 14026–14035.
Vinothkannan, M.; Kim, A. R.; Gnana Kumar, G.; Yoo, D. J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508.
Wang, H.; Sun, N.; Xu, X.; Wang, S.; Kang, W.; Zhuang, X.; Yin, Y.; Cheng, B. Adenosine triphosphate@graphene oxide proton channels for proton exchange membranes constructed via electrostatic layer-by-layer deposition. J. Membr. Sci. 2021, 620, 118880.
Yao, J.; Xu, G.; Zhao, Z.; Guo, J.; Li, S.; Cai, W.; Zhang, S. An enhanced proton conductivity and reduced methanol permeability composite membrane prepared by sulfonated covalent organic nanosheets/Nafion. Int. J. Hydrogen Energy 2019, 44, 24985–24996.
Prapainainar, P.; Pattanapisutkun, N.; Prapainainar, C.; Kongkachuichay, P. Incorporating graphene oxide to improve the performance of Nafion-mordenite composite membranes for a direct methanol fuel cell. Int. J. Hydrogen Energy 2019, 44, 362–378.
Al Munsur, A. Z.; Goo, B. H.; Kim, Y.; Kwon, O. J.; Paek, S. Y.; Lee, S. Y.; Kim, H. J.; Kim, T. H. Nafion-based proton-exchange membranes built on cross-linked semi-interpenetrating polymer networks between poly(acrylic acid) and poly(vinyl alcohol). ACS Appl. Mater. Interfaces 2021, 13, 28188–28200.
Ryu, S.; Lee, B.; Kim, J. H.; Pak, C.; Moon, S. H. High-temperature operation of PEMFC using pore-filling PTFE/Nafion composite membrane treated with electric field. Int. J. Energy Res. 2021, 45, 19136–19146.
Ru, C.; Gu, Y.; Duan, Y.; Zhao, C.; Na, H. Enhancement in proton conductivity and methanol resistance of Nafion membrane induced by blending sulfonated poly(arylene ether ketones) for direct methanol fuel cells. J. Membr. Sci. 2019, 573, 439–447.
Ru, C.; Gu, Y.; Duan, Y.; Na, H.; Zhao, C. Nafion based semi-interpenetrating polymer network membranes from a cross-linkable SPAEK and a fluorinated epoxy resin for DMFCs. Electrochim. Acta 2019, 324, 134873.
Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Yu, B. MXene as emerging nanofillers for high-performance polymer composites: a review. Compos. B Eng. 2021, 217, 108867.
Yang, Q.; Eder, S. J.; Martini, A.; Grützmacher, P. G. Effect of surface termination on the balance between friction and failure of Ti3C2Tx MXenes. NPJ Mater. Degrad. 2023, 7, 6.
Tunesi, M. M.; Soomro, R. A.; Han, X.; Zhu, Q.; Wei, Y.; Xu, B. Application of MXenes in environmental remediation technologies. Nano Converg. 2021, 8, 5.
Soleymaniha, M.; Shahbazi, M. A.; Rafieerad, A. R.; Maleki, A.; Amiri, A. Promoting role of MXene nanosheets in biomedical sciences: therapeutic and biosensing innovations. Adv. Healthc. Mater. 2019, 8, 1801137.
Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124.
Pandey, R. P.; Rasool, K.; Madhavan, V. E.; Aϊssa, B.; Gogotsi, Y.; Mahmoud, K. A. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533.
Zhou, H.; Han, S. J.; Lee, H. D.; Zhang, D.; Anayee, M.; Jo, S. H.; Gogotsi, Y.; Lee, T. W. Overcoming the limitations of MXene electrodes for solution-processed optoelectronic devices. Adv. Mater. 2022, 34, 2206377.
Wang, J.; Zhai, P.; Zhao, T.; Li, M.; Yang, Z.; Zhang, H.; Huang, J. Laminar MXene-Nafion-modified separator with highly inhibited shuttle effect for long-life lithium-sulfur batteries. Electrochim. Acta 2019, 320, 134558.
Jang, J.; Kang, Y.; Kim, K.; Kim, S.; Son, M.; Chee, S. S.; Kim, I. S. Concrete-structured Nafion@MXene/Cellulose acetate cation exchange membrane for reverse electrodialysis. J. Membr. Sci. 2022, 646, 120239.
Tang, X.; Zhou, Z.; Jiang, Y.; Wang, Q.; Sun, Q.; Zu, L.; Gao, X.; Lian, H.; Cao, M.; Cui, X. MXene enhanced the electromechanical performance of a Nafion-based actuator. Materials 2022, 15, 2833.
Lee, S. J.; Lee, D. H.; Lee, W. Y. An electrochemical sensor for capsaicin based on two-dimensional titanium carbide (MXene)-doped titania-Nafion composite film. Microchem. J. 2023, 185, 108216.
Guan, P.; Lei, J.; Zou, Y.; Zhang, Y. Improved thermo-mechanical properties and reduced hydrogen permeation of short side-chain perfluorosulfonic acid membranes doped with Ti3C2Tx. Materials 2021, 14, 7875.
Liu, Y.; Zhang, J.; Zhang, X.; Li, Y.; Wang, J. Ti3C2Tx filler effect on the proton conduction property of polymer electrolyte membrane. ACS Appl. Mater. Interfaces 2016, 8, 20352–20363.
Savage, J.; Tse, Y. L. S.; Voth, G. A. Proton transport mechanism of perfluorosulfonic acid membranes. J. Phys. Chem. C 2014, 118, 17436–17445.
Li, J.; Jin, S. H.; Lan, G. C.; Xu, Z. S.; Wang, L. T.; Wang, N.; Li, L. J. Research on the glass transition temperature and mechanical properties of poly(vinyl chloride)/dioctyl phthalate (PVC/DOP) blends by molecular dynamics simulations. Chinese J. Polym. Sci. 2019, 37, 834–840.
Wang, D. D.; Yu, K. F.; Xu, X. L.; Xu, W. S. Molecular dynamics study of star polymer melts under start-up shear. Chinese J. Polym. Sci. 2022, 40, 807–816.
Kritikos, G.; Pant, R.; Sengupta, S.; Karatasos, K.; Venkatnathan, A.; Lyulin, A. V. Nanostructure and dynamics of humidified Nafion/graphene-oxide composites via molecular dynamics simulations. J. Phys. Chem. C 2018, 122, 22864–22875.
Mabuchi, T.; Tokumasu, T. Relationship between proton transport and morphology of perfluorosulfonic acid membranes: a reactive molecular dynamics approach. J. Phys. Chem. B 2018, 122, 5922–5932.
Akbari, S.; Mosavian, M. T. H.; Moosavi, F.; Ahmadpour, A. Does the addition of a heteropoly acid change the water percolation threshold of PFSA membranes. Phys. Chem. Chem. Phys. 2019, 21, 25080–25089.
Savage, J.; Voth, G. A. Proton solvation and transport in realistic proton exchange membrane morphologies. J. Phys. Chem. C 2016, 120, 3176–3186.
Kuo, A. T.; Urata, S.; Nakabayashi, K.; Watabe, H.; Honmura, S. Coarse-grained molecular dynamics simulation of perfluorosulfonic acid polymer in water-ethanol mixtures. Macromolecules 2021, 54, 609–620.
Tarokh, A.; Karan, K.; Ponnurangam, S. Atomistic MD study of Nafion dispersions: role of solvent and counterion in the aggregate structure, ionic clustering, and acid dissociation. Macromolecules 2019, 53, 288–301.
Kuo, A. T.; Takeuchi, K.; Tanaka, A.; Urata, S.; Okazaki, S.; Shinoda, W. Exploring the effect of pendent side chain length on the structural and mechanical properties of hydrated perfluorosulfonic acid polymer membranes by molecular dynamics simulation. Polymer 2018, 146, 53–62.
Kuo, A. T.; Shinoda, W.; Okazaki, S. Molecular dynamics study of the morphology of hydrated perfluorosulfonic acid polymer membranes. J. Phys. Chem. C 2016, 120, 25832–25842.
Takeuchi, K.; Kuo, A. T.; Hirai, T.; Miyajima, T.; Urata, S.; Terazono, S.; Okazaki, S.; Shinoda, W. Hydrogen permeation in hydrated perfluorosulfonic acid polymer membranes: effect of polymer crystallinity and equivalent weight. J. Phys. Chem. C 2019, 123, 20628–20638.
Cui, R.; Li, S.; Yu, C.; Wang, Y.; Zhou, Y. Understanding the mechanism of nitrogen transport in the perfluorinated sulfonic-acid hydrated membranes via molecular dynamics simulations. J. Membr. Sci. 2022, 648, 120328.
Fan, L.; Xi, F.; Wang, X.; Xuan, J.; Jiao, K. Effects of side chain length on the structure, oxygen transport and thermal conductivity for perfluorosulfonic acid membrane: molecular dynamics simulation. J. Electrochem. Soc. 2019, 166, F511–F518.
Ban, S.; Huang, C.; Yuan, X. Z.; Wang, H. Molecular simulation of gas transport in hydrated Nafion membranes: influence of aqueous nanostructure. J. Phys. Chem. C 2012, 116, 17424–17430.
Ban, S.; Huang, C.; Yuan, X. Z.; Wang, H. Molecular simulation of gas adsorption, diffusion, and permeation in hydrated Nafion membranes. J. Phys. Chem. B 2011, 115, 11352–11358.
Cui, R.; Li, S.; Yu, C.; Zhou, Y. The evolution of hydrogen bond network in Nafion via molecular dynamics simulation. Macromolecules 2023, 56, 1688–1703.
Sun, S.; Ling, L.; Xiong, Y.; Zhang, Y.; Li, Z. Trifluoromethanesulfonimide-based hygroscopic semi-interpenetrating polymer network for enhanced proton conductivity of Nafion-based proton exchange membranes at low humidity. J. Membr. Sci. 2020, 612, 118339.
Atrazhev, V. V.; Astakhova, T. Y.; Sultanov, V. I.; Perry, M. L.; Burlatsky, S. F. Molecular dynamic study of water-cluster structure in PFSA and PFIA ionomers. J. Electrochem. Soc. 2017, 164, F1265–F1271.
Li, Z. Z.; Chen, L.; Tao, W. Q. Molecular dynamics simulation of water permeation through the Nafion membrane. Numer. Heat Tr. A-Appl. 2016, 70, 1232–1241.
Daly, K. B.; Benziger, J. B.; Debenedetti, P. G.; Panagiotopoulos, A. Z. Molecular dynamics simulations of water sorption in a perfluorosulfonic acid membrane. J. Phys. Chem. B 2013, 117, 12649–12660.
Gonçalves, W.; Mabuchi, T.; Tokumasu, T. Nucleation and growth of cavities in hydrated Nafion membranes under tensile strain: a molecular dynamics study. J. Phys. Chem. C 2019, 123, 28958–28968.
Kuo, A. T.; Miyazaki, Y.; Jang, C.; Miyajima, T.; Urata, S.; Nielsen, S. O.; Okazaki, S.; Shinoda, W. Large-scale molecular dynamics simulation of perfluorosulfonic acid membranes: remapping coarse-grained to all-atomistic simulations. Polymer 2019, 181, 121766.
Kuo, A. T.; Tanaka, A.; Irisawa, J.; Shinoda, W.; Okazaki, S. Molecular dynamics study on the mechanical deformation of hydrated perfluorosulfonic acid polymer membranes. J. Phys. Chem. C 2017, 121, 21374–21382.
Xie, J.; Ban, S.; Liu, B.; Zhou, H. A molecular simulation study of chemical degradation and mechanical deformation of hydrated Nafion membranes. Appl. Surf. Sci. 2016, 362, 441–447.
Maiti, T. K.; Singh, J.; Maiti, S. K.; Majhi, J.; Ahuja, A.; Singh, M.; Bandyopadhyay, A.; Manik, G.; Chattopadhyay, S. Molecular dynamics simulations and experimental studies of the perfluorosulfonic acid-based composite membranes containing sulfonated graphene oxide for fuel cell applications. Eur. Polym. J. 2022, 174, 111345.
Haghighi Asl, M.; Moosavi, F.; Akbari, S. Mixed membrane matrices (MMMs) based on Nafion® pristine/defected-UiO-66(Zr) MOFs: assessment of the effects of dopants on cluster morphology. Mol. Syst. Des. Eng. 2022, 7, 969–985.
Liu, Y.; Sambasivarao, S. V.; Horan, J. L.; Yang, Y.; Maupin, C. M.; Herring, A. M. A combined theoretical and experimental investigation of the transport properties of water in a perfluorosulfonic acid proton exchange membrane doped with the heteropoly acids, H3PW12O40 or H4SiW12O40. J. Phys. Chem. C 2013, 118, 854–863.
Sambasivarao, S. V.; Liu, Y.; Horan, J. L.; Seifert, S.; Herring, A. M.; Maupin, C. M. Enhancing proton transport and membrane lifetimes in perfluorosulfonic acid proton exchange membranes: a combined computational and experimental evaluation of the structure and morphology changes due to H3PW12O40 doping. J. Phys. Chem. C 2014, 118, 20193–20202.
Akbari, S.; Hamed Mosavian, M. T.; Moosavi, F.; Ahmadpour, A. Elucidating the morphological aspects and proton dynamics in a hybrid perfluorosulfonic acid membrane for medium-temperature fuel cell applications. Phys. Chem. Chem. Phys. 2018, 20, 29778–29789.
Akbari, S.; Hamed Mosavian, M. T.; Moosavi, F.; Ahmadpour, A. Atomistic simulation of proton transfer ability of isopoly acid (IPA)/heteropoly acid (HPA) doped Nafion® 117 for high-temperature fuel cell applications. Compos. B Eng. 2019, 161, 402–410.
Lee, O. S.; Madjet, M. E.; Mahmoud, K. A. Antibacterial mechanism of multifunctional MXene nanosheets: domain formation and phase transition in lipid bilayer. Nano Lett. 2021, 21, 8510–8517.
Hope, M. A.; Forse, A. C.; Griffith, K. J.; Lukatskaya, M. R.; Ghidiu, M.; Gogotsi, Y.; Grey, C. P. NMR reveals the surface functionalisation of Ti3C2 MXene. Phys. Chem. Chem. Phys. 2016, 18, 5099–5102.
Martinez, L.; Andrade, R.; Birgin, E. G.; Martinez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164.
Wang, C.; Clark, J. K.; Kumar, M.; Paddison, S. J. An ab initio study of the primary hydration and proton transfer of CF3SO3H and CF3O(CF2)2SO3H: effects of the hybrid functional and inclusion of diffuse functions. Solid State Ion. 2011, 199–200, 6–13.
Mabuchi, T.; Tokumasu, T. Effect of bound state of water on hydronium ion mobility in hydrated Nafion using molecular dynamics simulations. J. Chem. Phys. 2014, 141, 104904.
Levitt, M.; Hirshberg, M.; Sharon, R.; Laidig, K. E.; Daggett, V. Calibration and testing of a water model for simulation of the molecular dynamics of proteins and nucleic acids in solution. J. Phys. Chem. B 1997, 101, 5051–5061.
Jang, S. S.; Molinero, V.; Çagin, T.; Goddard, W. A. Nanophase-segregation and transport in Nafion 117 from molecular dynamics simulations: effect of monomeric sequence. J. Phys. Chem. B 2004, 108, 3149–3157.
Rappe, A. K.; Casewit, C. J.; Colwell, K. S.; Goddard, W. A.; Skiff, W. M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 2002, 114, 10024–10035.
Hu, T.; Wang, J.; Zhang, H.; Li, Z.; Hu, M.; Wang, X. Vibrational properties of Ti3C2 and Ti3C2T2 (T = O, F, OH) monosheets by first-principles calculations: a comparative study. Phys. Chem. Chem. Phys. 2015, 17, 9997–10003.
Xu, K.; Lin, Z.; Merlet, C.; Taberna, P. L.; Miao, L.; Jiang, J.; Simon, P. Tracking ionic rearrangements and interpreting dynamic volumetric changes in two-dimensional metal carbide supercapacitors: a molecular dynamics simulation study. ChemSusChem 2018, 11, 1892–1899.
Abraham, M. J.; Murtola, T.; Schulz, R.; Pall, S.; Smith, J. C.; Hess, B.; Lindahl, E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. Softwarex 2015, 1–2, 19–25.
Martyna, G. J.; Klein, M. L.; Tuckerman, M. Nosé-Hoover chains: the canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643.
Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190.
Essmann, U.; Perera, L.; Berkowitz, M. L.; Darden, T.; Lee, H.; Pedersen, L. G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593.
Liu, W.; Hong, G.; Dai, D.; Li, L.; Dolg, M. The Beijing four-component density functional program package (BDF) and its application to EuO, EuS, YbO and YbS. Theor. Chem. Acc. 1997, 96, 75–83.
Zhang, Y.; Suo, B.; Wang, Z.; Zhang, N.; Li, Z.; Lei, Y.; Zou, W.; Gao, J.; Peng, D.; Pu, Z.; Xiao, Y.; Sun, Q.; Wang, F.; Ma, Y.; Wang, X.; Guo, Y.; Liu, W. BDF: a relativistic electronic structure program package. J. Chem. Phys. 2020, 152, 064113.
Liu, W. J.; Wang, F.; Li, L. M. The Beijing Density Functional (BDF) program package: methodologies and applications. J. Theor. Comput. Chem. 2003, 2, 257–272.
Liu, W. J.; Wang, F.; Li, L. Relativistic Density Functional Theory: The BDF Program Package. World Scientific, Singapore, 2004, p. 257–282.
Wang, Z.; Li, Z.; Zhang, Y.; Liu, W. Analytic energy gradients of spin-adapted open-shell time-dependent density functional theory. J. Chem. Phys. 2020, 153, 164109.
Hongzhiwei Technology, Device Studio, Version 2023A, China, 2023. Available online: https://cloud.hzwtech.com/web/product-service?id=6 (accessed on Aug., 23rd).
Morris, D. R.; Sun, X. Water-sorption and transport properties of Nafion 117. J. Appl. Polym. Sci. 1993, 50, 1445–1452.
Weber, A. Z.; Newman, J. Transport in polymer-electrolyte membranes: II. Mathematical model. J. Electrochem. Soc. 2004, 151, A311–A325.
Ohkubo, T.; Kidena, K.; Takimoto, N.; Ohira, A. Molecular dynamics simulations of Nafion and sulfonated polyether sulfone membranes. I. Effect of hydration on aqueous phase structure. J. Mol. Model. 2011, 17, 739–755.
Liu, J.; Suraweera, N.; Keffer, D. J.; Cui, S.; Paddison, S. J. On the relationship between polymer electrolyte structure and hydrated morphology of perfluorosulfonic acid membranes. J. Phys. Chem. C 2010, 114, 11279–11292.
Dixit, S.; Crain, J.; Poon, W. C.; Finney, J. L.; Soper, A. K. Molecular segregation observed in a concentrated alcohol-water solution. Nature 2002, 411, 829–832.
Zhou, A.; Liu, Y.; Li, S.; Wang, X.; Ying, G.; Xia, Q.; Zhang, P. From structural ceramics to 2D materials with multi-applications: a review on the development from MAX phases to MXenes. J. Adv. Ceram. 2021, 10, 1194–1242.
Devanathan, R.; Venkatnathan, A.; Rousseau, R.; Dupuis, M.; Frigato, T.; Gu, W.; Helms, V. Atomistic simulation of water percolation and proton hopping in Nafion fuel cell membrane. J. Phys. Chem. B 2010, 114, 13681–13690.
Zawodzinski, T. A.; Neeman, M.; Sillerud, L. O.; Gottesfeld, S. Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J. Phys. Chem. 1991, 95, 6040–6044.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (Nos. 2020YFB1505500 and 2020YFB1505503). We gratefully acknowledge HZWTECH for providing computation facilities. C. Y. thanks Shuoqi Sun for help and discussions regarding this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no interest conflict.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Han, ZY., Pei, SP., Yu, CY. et al. Molecular Dynamics Simulation Studies on the Micromorphology and Proton Transport of Nafion/Ti3C2Tx Composite Membrane. Chin J Polym Sci 42, 373–387 (2024). https://doi.org/10.1007/s10118-024-3063-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-024-3063-2