Abstract
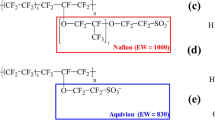
We measured the water uptakes and proton conductivities of a Nafion membrane and three sulfonated polyether sulfone membranes (SPESs) with different values of ion-exchange capacity (IEC = 0.75, 1.0 and 1.4 meq/g) in relation to relative humidity in order to apply the findings to polymer electrolyte membrane fuel cells. The number of water molecules per sulfonic acid group λ at each humidity level was independent of the relative humidity for all membranes, but the proton conductivities of the SPESs were inferior to that of Nafion for the same λ value. Classical molecular dynamics simulations for the same membranes were carried out using a consistent force field at λ = 3, 6, 9, 12 and 15. The structural properties of water molecules and hydronium ions at a molecular level were estimated from radial distribution functions and cluster size distributions of water. We found that the radial distribution function of S(sulfonic acid)–S(sulfonic acid) of Nafion at λ = 3 indicated a significant correlation between the S–S pair, due to water channels, while the S–S pair of the SPESs showed a poor correlation. The cluster size distribution of water was also calculated in order to estimate the connectivity of the water channel. It is clear that some water is present in the SPESs as small, isolated clusters, especially when the water content is low.













Similar content being viewed by others
References
Carrette L, Friedrich KA, Stimming U (2001) Fuel cells—fundamentals and applications. Fuel Cells 1:5–39
Doyle M, Rajendran G (2003) Perfluorinated membranes in fuel cell technology and applications. In: Vielstich W, Lamm A, Gasteiger HA (eds) Handbook of fuel cells, vol. 3. Wiley, Chichester, pp 351–395
Paddison SJ (2003) First principles modeling of sulfonic acid based ionomer membranes in fuel cell technology and applications. In: Vielstich W, Lamm A, Gasteiger HA (eds) Handbook of fuel cells, vol. 3. Wiley, Chichester, pp 396–411
Nakano M, Yoshitake M (2003) Composite perfluorinate membranes in fuel cell technology and applications. In: Vielstich W, Lamm A, Gasteiger HA (eds) Handbook of fuel cells, vol. 3. Wiley, Chichester, pp 412–419
Kreuer KD (2003) Hydrocarbon membranes in fuel cell technology and applications. In: Vielstich W, Lamm A, Gasteiger HA (eds) Handbook of fuel cells, vol. 3. Wiley, Chichester, pp 420–435
Zawodzinski TA, Neeman M, Sillerud LO, Gottesfeld S (1991) J Phys Chem B 95:6040–6047
Saito M, Tsuzuki S, Hayamizu K, Okada T (2006) J Phys Chem B 110:24410–24417
Yeo RS (1983) J Electrochem Soc 130:533–538
Uosaki K, Okazaki K, Kita H (1990) J Electrochem Soc 287:163–169
Thompson EL, Capehart TW, Fuller TJ, Jorne J (2006) J Electrochem Soc 153:A2351–A2362
Mohameda HFM, Ito K, Kobayashi Y, Takimoto N, Takeoka Y, Ohira A (2008) Polymer 49:3091–3097
Ogumi Z, Takehara Z, Yoshizawa S (1984) J Electrochem Soc 131:769–773
Ogumi Z, Takehara Z, Yoshizawa S (1985) J Electrochem Soc 132:2601–2605
Büchi FN, Wakizoe M, Srinivasan S (1996) J Electrochem Soc 143:2601–2605
Zawodzinski TA, Derouin C, Radzinski S, Sherman RJ, Smith VT, Springer TE, Gottesfeld S (1993) J Electrochem Soc 140:1041–1047
Kinumoto T, Inaba M, Nakayama Y, Ogata K, Umebayashi R, Tasaka A, Iriyama Y, Abe T, Ogumi Z (2006) J Power Sources 158:1222–1228
Pozio A, Silva RF, Francesco M, Giorgi L (2003) Electrochim Acta 48:1543–1549
Aoki M, Uchida H, Watanabe M (2005) Electrochem Commun 7:1434–1438
Zaidi SMJ, Mikhailenko SD, Robertson GP, Guiver MD, Kaliaguine S (2000) J Membr Sci 173:17–34
Gil M, Ji XL, Li XF, Na H, Hampsey JE, Lu YF (2004) J Membr Sci 234:75–81
Li L, Zhang J, Wang YX (2003) J Membr Sci 226:159–167
Hickner MA, Ghassemi H, Kim YS, Einsla BR, McGrath JE (2004) Chem Rev 104:4587–4611
Mauritz KA, Moore RB (2004) Chem Rev 104:4535–4585
HeitnerWirguin C (1996) J Membr Sci 120:1–33
Kreuer KD, Paddison SJ, Spohr E, Schuster M (2004) Chem Rev 104:4637–4678
Kim MH, Glinka CJ, Grot SA, Grot WG (2006) Macromolecules 39:4775–4787
Schmidt-Rohr K, Chen Q (2008) Nat Mater 7:75–83
Rubatat L, Rollet AL, Gebel G, Diat O (2002) Macromolecules 35:4050–4055
Gebel G, Lambard J (1997) Macromolecules 30:7914–7920
Paddison SJ, Elliott JA (2005) J Phys Chem A 109:7583–7593
Paddison SJ, Zawodzinski TA (1998) Solid State Ion 113:333–340
Eikerling M, Paddison SJ, Pratt LR, Zawodzinski TA (2003) Chem Phys Lett 368:108–114
Roudgar A, Narasimachary SP, Eikerling M (2008) Chem Phys Lett 457:337–341
Cui S, Liu J, Selvan ME, Keffer DJ, Edwards BJ, Steele WV (2007) J Phys Chem B 111:2208–2218
Cui S, Liu J, Selvan ME, Paddison SJ, Keffer DJ, Edwards BJ (2008) J Phys Chem B 112:13273–13284
Devanathan R, Venkatnathan A, Dupuis M (2007) J Phys Chem B 111:8069–8079
Devanathan R, Venkatnathan A, Dupuis M (2007) J Phys Chem B 111:13006–13013
Venkatnathan A, Devanathan R, Dupuis M (2007) J Phys Chem B 111:7234–7244
Jang SS, Molinero V, Cagin T, Goddard WA (2004) J Phys Chem B 108:3149–3157
Brandell D, Karo J, Liivat A, Thomas JO (2007) J Mol Model 13:1039–1046
Urata S, Irisawa J, Takada A, Shinoda W, Tsuzuki S, Mikami M (2005) J Phys Chem B 109:4269–4278
Kim YS, Wang F, Hickner M, McCartney S, Hong YT, Harrison W, Zawodzinski TA, McGrath JE (2003) J Polym Sci Pt B 41:2816–2828
Kobayashi T, Rikukawa M, Sanui K, Ogata N (1998) Solid State Ion 106:219–225
Kim YS, Wang F, Hickner M, Zawodzinski TA, McGrath JE (2003) J Membr Sci 212:263–282
Cho CG, Kim YS, Yu X, Hill M, McGrath JE (2006) J Polym Sci Pol Chem 44:6007–6014
Sethuraman VA, Weidner JW, Haug AT, Protsailo LV (2008) J Electrochem Soc 155:B119–B124
Roy A, Hickner MA, Einsla BR, Harrison WL, Mcgrath JE (2009) J Polym Sci Pol Chem 47:384–391
Scienomics SARL (2002) MAPS, version 3.1. Scienomics SARL, Paris
Maple JR, Hwang MJ, Stockfisch TP, Dinur U, Waldman M, Ewig CS, Hagler AT (1994) J Comput Chem 15:162–182
Sun H (1998) J Phys Chem B 102:7338–7364
Pozuelo J, Riande E, Saiz E, Compan V (2006) Macromolecules 25:8862–8866
Hu N, Chen R, Hsu A (2006) Polym Int 55:872–882
Aeon Technology, Inc. (2002) Direct Force Field, version 6.0. Aeon Technology, Inc., San Diego
Sato F, Hojo S, Sun H (2003) J Phys Chem A 107:248–257
Becke AD (1993) J Chem Phys 98:5648–5652
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Miehlich B, Savin A, Stoll H, Preuss H (1989) Chem Phys Lett 157:200–206
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265–3269
Chirlian LE, Francl MM (1987) J Comput Chem 8:894–905
Plimpton S (1995) J Comput Phys 117:1–19
Verlet L (1967) Phys Rev 159:98–103
Hockney RW, Eastwood JW (1981) Computer simulation using particles. McGraw-Hill, New York
Nose S (1986) Mol Phys 57:187–191
Nose S (1984) J Chem Phys 81:511–519
Nose S (1984) Mol Phys 52:255–268
Nose S, Klein ML (1983) J Chem Phys 78:6928–6939
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14:33
Paddison SJ, Elliott JA (2006) Phys Chem Chem Phys 8:2193–2203
Paddison SJ, Elliott JA (2006) Solid State Ion 177:2385–2390
Acknowledgements
We thank F. Sato for helpful discussions on the development of force fields. We are grateful to A. Yashiro of Sumitomo Chemical Co., Ltd. for providing SPES samples. This work was supported by the New Energy Development Organization (NEDO), Japan.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Force field parameters
Rights and permissions
About this article
Cite this article
Ohkubo, T., Kidena, K., Takimoto, N. et al. Molecular dynamics simulations of Nafion and sulfonated polyether sulfone membranes. I. Effect of hydration on aqueous phase structure. J Mol Model 17, 739–755 (2011). https://doi.org/10.1007/s00894-010-0767-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0767-8