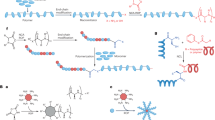
Abstract
Antimicrobial peptides (AMPs) have been considered as an alternative to small molecule antibiotics since they are difficult to develop antimicrobial resistance. Hyperbranched polylysine (HPL), an AMP mimics, has gained attention due to its broad-spectrum antibacterial activities, but it also suffers from high toxicity. Here we report a facile strategy to engineer the toxicity of HPL by copolymerizing lysine (K) with a hydrophobic amino acid, e.g., alanine (A), tryptophan (W) or phenylalanine (F), to afford hyperbranched random copolymers. These copolymers have comparable antibacterial activities to HPL while their cytotoxicities and in vivo toxicities are lowered when the type and content of hydrophobic amino acid and the size of copolymers are optimized. The G. mellonella infection model demonstrates that the copolymers are effective against the S. aureus infection in vivo. The copolymers kill the bacteria through the disruption of cell membranes and the bacteria do not develop resistance to the copolymers.
Similar content being viewed by others
References
Hutchings, M. I.; Truman, A. W.; Wilkinson, B. Antibiotics: past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80.
Broughton, C. E.; Van Den Berg, H. A.; Wemyss, A. M.; Roper, D. I.; Rodger, A. Beyond the discovery void: new targets for antibacterial compounds. Sci. Prog. 2016, 99, 153–182.
Shen, S.; Huang, Y.; Yuan, A.; Lv, F.; Liu, L.; Wang, S. Electrochemical regulation of antibacterial activity using ferrocene-containing antibiotics. CCS Chem. 2021, 3, 129–135.
Duval, R. E.; Grare, M.; Demore, B. Fight against antimicrobial resistance: we always need new antibacterials but for right bacteria. Molecules 2019, 24, 9.
Blaskovich, M. A. T. The fight against antimicrobial resistance is confounded by a global increase in antibiotic usage. ACS Infect. Dis. 2018, 4, 868–870.
Luong, H. X.; Thanh, T. T.; Tran, T. H. Antimicrobial peptides-advances in development of therapeutic applications. Life Sci. 2020, 260, 118407.
Xie, X.; Gao, B.; Ma, Z.; Liu, J.; Zhang, J.; Liang, J.; Chen, Z.; Wu, L.; Li, W. Host-guest interaction driven peptide assembly into photoresponsive two-dimensional nanosheets with switchable antibacterial activity. CCS Chem. 2021, 3, 1949–1962.
Cruz, J.; Ortiz, C.; Guzman, F.; Fernandez-Lafuente, R.; Torres, R. Antimicrobial peptides: promising compounds against pathogenic microorganisms. Curr. Med. Chem. 2014, 21, 2299–2321.
Hancock, R. E. W.; Sahl, H. G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557.
Da Costa, J. P.; Cova, M.; Ferreira, R.; Vitorino, R. Antimicrobial peptides: an alternative for innovative medicines. Appl. Microbiol. Biot. 2015, 99, 2023–2040.
Wang, J. J.; Dou, X. J.; Song, J.; Lyu, Y. F.; Zhu, X.; Xu, L.; Li, W. Z.; Shan, A. S. Antimicrobial peptides: promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859.
Kawano, Y.; Jordan, O.; Hanawa, T.; Borchard, G.; Patrulea, V. Are antimicrobial peptide dendrimers an Escape from ESKAPE. Adv. Wound Care 2020, 9, 378–395.
Ciornei, C. D.; Sigurdardottir, T.; Schmidtchen, A.; Bodelsson, M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob. Agents Chemother. 2005, 49, 2845–2850.
Hartlieb, M.; Williams, E. G. L.; Kuroki, A.; Perrier, S.; Locock, K. E. S. Antimicrobial polymers: mimicking amino acid functionality, sequence control and three-dimensional structure of host-defense peptides. Curr. Med. Chem. 2017, 24, 2115–2140.
Salas-Ambrosio, P.; Tronnet, A.; Verhaeghe, P.; Bonduelle, C. Synthetic polypeptide polymers as simplified analogues of antimicrobial peptides. Biomacromolecules 2021, 22, 57–75.
Shen, W.; He, P.; Xiao, C.; Chen, X. From antimicrobial peptides to antimicrobial poly(alpha-amino acid)s. Adv. Healthc. Mater. 2018, 7, 1800354.
Shima, S.; Matsuoka, H.; Iwamoto, T.; Sakai, H. Antibacterial action of epsilon-poly-L-lysine. J. Antibiot. 1984, 37, 1449–1455.
Katchalski, E.; Grossfeld, I.; Frankel, M. Poly-condensation of alpha-amino acid derivatives. 3. Poly-lysine. J. Am. Chem. Soc. 1948, 70, 2094–2101.
Katchalski, E.; Bichowskislomnitzki, L.; Volcani, B. E. The action of some water-soluble poly-alpha-amino acids on bacteria. Biochem. J. 1953, 55, 671–680.
Mayandi, V.; Xi, Q. X.; Goh, E. T. L.; Koh, S. K.; Toh, T. Y. J.; Barathi, V. A.; Fazil, M.; Chalasani, M. L. S.; Varadarajan, J.; Ting, D. S. J.; Beuerman, R. W.; Chan, L. W.; Agrawal, R.; Barkham, T. M. S.; Zhou, L.; Verma, N. K.; Lakshminarayanan, R. Rational substitution of epsilon-lysine for alpha-lysine enhances the cell and membrane selectivity of pore-forming melittin. J. Med. Chem. 2020, 63, 3522–3537.
Zhou, C. C.; Qi, X. B.; Li, P.; Chen, W. N.; Mouad, L.; Chang, M. W.; Leong, S. S. J.; Chan-Park, M. B. High potency and broad-spectrum antimicrobial peptides synthesized via ring-opening polymerization of alpha-aminoacid-N-carboxyanhydrides. Biomacromolecules 2010, 11, 60–67.
Zhou, X.; Su, X.; Tan, Z.; Zhou, C. Synthesis of triblock amphiphilic copolypeptides with excellent antibacterial activity. Eur. Polym. J. 2018, 106, 175–181.
Lam, S. J.; O’Brien-Simpson, N. M.; Pantarat, N.; Sulistio, A.; Wong, E. H.; Chen, Y. Y.; Lenzo, J. C.; Holden, J. A.; Blencowe, A.; Reynolds, E. C.; Qiao, G. G. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162.
Chen, Y. F.; Lai, Y. D.; Chang, C. H.; Tsai, Y. C.; Tang, C. C.; Jan, J. S. Star-shaped polypeptides exhibit potent antibacterial activities. Nanoscale 2019, 11, 11696–11708.
Chen, S. J.; Huang, S. T.; Li, Y.; Zhou, C. C. Recent advances in epsilon-poly-L-lysine and L-lysine-based dendrimer synthesis, modification, and biomedical applications. Front. Chem. 2021, 9, 14.
Hyldgaard, M.; Mygind, T.; Vad, B. S.; Stenvang, M.; Otzen, D. E.; Meyer, R. L. The antimicrobial mechanism of action of epsilon-poly-L-lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770.
Zou, Y. J.; He, S. S.; Du, J. Z. Epsilon-poly(L-lysine)-based hydrogels with fast-acting and prolonged antibacterial activities. Chinese J. Polym. Sci. 2018, 36, 1239–1250.
Shi, C.; He, Y.; Feng, X. B.; Fu, D. H. Epsilon-polylysine and next-generation dendrigraft poly-L-lysine: chemistry, activity, and applications in biopharmaceuticals. J. Biomater. Sci., Polym. Ed. 2015, 26, 1343–1356.
Liu, X.; Guo, J. W.; Liu, Y. D.; Liu, M.; Liu, H.; Han, M. M.; Ji, S. X. Antibacterial thermoplastic polyurethane/PL-DOSS composite films. Chinese J. Polym. Sci. 2021, 39, 1020–1028.
Ye, R.; Xu, H.; Wan, C.; Peng, S.; Wang, L.; Xu, H.; Aguilar, Z. P.; Xiong, Y.; Zeng, Z.; Wei, H. Antibacterial activity and mechanism of action of ε-poly-L-lysine. Biochem. Biophys. Res. Commun. 2013, 439, 148–153.
Shukla, S. C.; Singh, A.; Pandey, A. K.; Mishra, A. Review on production and medical applications of ε-polylysine. Biochem. Eng. J. 2012, 65, 70–81.
Harada, K. Thermal homopolymerization of lysine and copolymerization with neutral and acidic amino acids. Bull. Chem. Soc. Jpn. 1959, 32, 1007–1008.
Harada K; W., F. S. Characterization of thermal polymers of neutral α-amino acids with dicarboxylic amino acids or lysine. Arch. Biochem. Biophys. 1965, 109, 49–56.
Scholl, M.; Kadlecova, Z.; Klok, H. A. Dendritic and hyperbranched polyamides. Prog. Polym. Sci. 2009, 34, 24–61.
Liu, X.; Yang, Y.; Han, M.; Guo, J.; Liu, H.; Liu, Y.; Xu, J.; Ji, S.; Chen, X. Guanylated hyperbranched polylysines with high in vitro and in vivo antifungal activity. Adv. Healthc. Mater. 2022, 2201091.
Kadlecova, Z.; Rajendra, Y.; Matasci, M.; Baldi, L.; Hacker, D. L.; Wurm, F. M.; Klok, H. A. DNA delivery with hyperbranched polylysine: a comparative study with linear and dendritic polylysine. J. Controlled Release 2013, 169, 276–288.
Kadlecova, Z.; Baldi, L.; Hacker, D.; Wurm, F. M.; Klok, H. A. Comparative study on the in vitro cytotoxicity of linear, dendritic, and hyperbranched polylysine analogues. Biomacromolecules 2012, 13, 3127–3137.
Thompson, M.; Scholz, C. Highly branched polymers based on poly(amino acid)s for biomedical application. Nanomaterials 2021, 11, 23.
Ramarao, N.; Nielsen-Leroux, C.; Lereclus, D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. JoVE 2012, 70, 4392.
Balouiri, M.; Sadiki, M.; Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 2016, 6, 71–79.
Fischer, W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med. Microbiol. Immunol. 1994, 183, 61–76.
Beveridge, T. J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733.
Li, P.; Zhou, C.; Rayatpisheh, S.; Ye, K.; Poon, Y. F.; Hammond, P. T.; Duan, H.; Chan-Park, M. B. Cationic peptidopolysaccharides show excellent broad-spectrum antimicrobial activities and high selectivity. Adv. Mater. 2012, 24, 4130–4137.
Lu, C.; Quan, G. L.; Su, M.; Nimmagadda, A.; Chen, W. D.; Pan, M.; Teng, P.; Yu, F. Y.; Liu, X.; Jiang, L.; Du, W. Y.; Hu, W.; Yao, F.; Pan, X.; Wu, C. B.; Liu, D. J.; Cai, J. F. Molecular architecture and charging effects enhance the in vitro and in vivo performance of multi-arm antimicrobial agents based on star-shaped poly(L-lysine). Adv. Ther. 2019, 2, 11.
Yeaman, M. R.; Yount, N. Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55.
Nam, S. Y.; Lee, J.; Shin, S. S.; Yoo, H. J.; Yun, M.; Kim, S.; Kim, J. H.; Lee, J. H. Antibacterial and cytotoxic properties of star-shaped quaternary ammonium-functionalized polymers with different pendant groups. Polym. Chem. 2022, 13, 1763–1773.
Al-Badri, Z. M.; Som, A.; Lyon, S.; Nelson, C. F.; Nusslein, K.; Tew, G. N. Investigating the effect of increasing charge density on the hemolytic activity of synthetic antimicrobial polymers. Biomacromolecules 2008, 9, 2805–2810.
Palermo, E. F.; Kuroda, K. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biot. 2010, 87, 1605–1615.
Li, P.; Poon, Y. F.; Li, W.; Zhu, H.-Y.; Yeap, S. H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R. W.; Kang, E.-T.; Mu, Y.; Li, C. M.; Chang, M. W.; Jan Leong, S. S.; Chan-Park, M. B. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2011, 10, 149–156.
Eisenberg, D.; Weiss, R. M.; Terwilliger, T. C.; Wilcox, W. Hydrophobic moments and protein-structure. Faraday Symp. Chem. Soc. 1982, 17, 109–120.
Van den Bergen, G.; Stroet, M.; Caron, B.; Poger, D.; Mark, A. E. Curved or linear? Predicting the 3-dimensional structure of α-helical antimicrobial peptides in an amphipathic environment. FEBS Lett. 2020, 594, 1062–1080.
Su, X.; Zhou, X.; Tan, Z.; Zhou, C. Highly efficient antibacterial diblock copolypeptides based on lysine and phenylalanine. Biopolymers 2017, 107, 23041.
Ramamourthy, G.; Park, J.; Seo, C.; Vogel, H. J.; Park, Y. Antifungal and antibiofilm activities and the mechanism of action of repeating lysine-tryptophan peptides against Candida albicans. Microorganisms 2020, 8, 22.
Kuroda, K.; Caputo, G. A.; DeGrado, W. F. The role of hydrophobicity in the antimicrobial and hemolytic activities of polymethacrylate derivatives. Chem. Eur. J. 2009, 15, 1123–1133.
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395.
Lienkamp, K.; Madkour, A. E.; Kumar, K. N.; Nüsslein, K.; Tew, G. N. Antimicrobial polymers prepared by ring-opening metathesis polymerization: manipulating antimicrobial properties by organic counterion and charge density variation. Chem. Eur. J. 2009, 15, 11715–11722.
Chen, L. C.; Kung, S. K.; Chen, H. H.; Lin, S. B. Evaluation of zeta potential difference as an indicator for antibacterial strength of low molecular weight chitosan. Carbohydr. Polym. 2010, 82, 913–919.
Henkelman, S.; Rakhorst, G.; Blanton, J.; van Oeveren, W. Standardization of incubation conditions for hemolysis testing of biomaterials. Mater. Sci. Eng., C 2009, 29, 1650–1654.
Ong, Z. Y. I.; Yang, C.; Cheng, W.; Voo, Z. X.; Chin, W.; Hedrick, J. L.; Yang, Y. Y. Biodegradable cationic poly(carbonates): effect of varying side chain hydrophobicity on key aspects of gene transfection. Acta Biomater. 2017, 54, 201–211.
Chen, J.; Jiao, Z. X.; Lin, L.; Guo, Z. P.; Xu, C. N.; Li, Y. H.; Tian, H. Y.; Chen, X. S. Polylysine-modified polyethylenimines as siRNA carriers for effective tumor treatment. Chinese J. Polym. Sci. 2015, 33, 830–837.
Monnery, B. D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J. H. G.; Thanou, M. Cytotoxicity of polycations: relationship of molecular weight and the hydrolytic theory of the mechanism of toxicity. Int. J. Pharm. 2017, 521, 249–258.
Huang, M.; Khor, E.; Lim, L. Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353.
Colomer, A.; Pinazo, A.; Garcia, M. T.; Mitjans, M.; Vinardell, M. P.; Infante, M. R.; Martinez, V.; Perez, L. pH-sensitive surfactants from lysine: assessment of their cytotoxicity and environmental behavior. Langmuir 2012, 28, 5900–5912.
Uppu, D.; Samaddar, S.; Hoque, J.; Konai, M. M.; Krishnamoorthy, P.; Shome, B. R.; Haldar, J. Side chain degradable cationic-amphiphilic polymers with tunable hydrophobicity show in vivo activity. Biomacromolecules 2016, 17, 3094–3102.
Choksakulnimitr, S.; Masuda, S.; Tokuda, H.; Takakura, Y.; Hashida, M. In vitro cytotoxicity of macromolecules in different cell culture systems. J. Controlled Release 1995, 34, 233–241.
Morgan, D. M.; Clover, J.; Pearson, J. D. Effects of synthetic polycations on leucine incorporation, lactate dehydrogenase release, and morphology of human umbilical vein endothelial cells. J. Cell Sci. 1988, 91, 231–238.
Morgan, D. M.; Larvin, V. L.; Pearson, J. D. Biochamical characterisation of polycation-induced cytotoxicity to human vascular endothelial cells. J. Cell Sci. 1989, 94, 553–559.
Frederiksen, N.; Hansen, P. R.; Zabicka, D.; Tomczak, M.; Urbas, M.; Domraceva, I.; Björkling, F.; Franzyk, H. Alternating cationic-hydrophobic peptide/peptoid hybrids: influence of hydrophobicity on antibacterial activity and cell selectivity. ChemMedChem 2020, 15, 2544–2561.
Lee, J.; Kang, D.; Choi, J.; Huang, W.; Wadman, M.; Barron, A. E.; Seo, J. Effect of side chain hydrophobicity and cationic charge on antimicrobial activity and cytotoxicity of helical peptoids. Bioorg. Med. Chem. Lett. 2018, 28, 170–173.
Lienkamp, K.; Kumar, K. N.; Som, A.; Nüsslein, K.; Tew, G. N. “Doubly selective” antimicrobial polymers: how do they differentiate between bacteria. Chem. Eur. J. 2009, 15, 11710–11714.
Konaté, K.; Mavoungou, J. F.; Lepengué, A. N.; Aworet-Samseny, R. R. R.; Hilou, A.; Souza, A.; Dicko, M. H.; M’Batchi, B. Antibacterial activity against β-lactamase producing Methicillin and Ampicillin-resistants Staphylococcus aureus: fractional Inhibitory Concentration Index (FICI) determination. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 18.
Kavanagh, K.; Reeves, E. P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 101–112.
Desbois, A. P.; Coote, P. J. Wax moth larva (Galleria mellonella): an in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011, 66, 1785–1790.
Gibreel, T. M.; Upton, M. Synthetic epidermicin NI01 can protect Galleria mellonella larvae from infection with Staphylococcus aureus. J. Antimicrob. Chemother. 2013, 68, 2269–2273.
Brackman, G.; Cos, P.; Maes, L.; Nelis, H. J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661.
Peleg, A. Y.; Monga, D.; Pillai, S.; Mylonakis, E.; Moellering, R. C.; Eliopoulos, G. M. Reduced susceptibility to vancomycin influences pathogenicity in Staphylococcus aureus infection. J. Infect. Dis. 2009, 199, 532–536.
Gao, W.; Chua, K.; Davies, J. K.; Newton, H. J.; Seemann, T.; Harrison, P. F.; Holmes, N. E.; Rhee, H. W.; Hong, J. I.; Hartland, E. L.; Stinear, T. P.; Howden, B. P. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent Infection. PLoS Pathog. 2010, 6, 100094.
Peleg, A. Y.; Jara, S.; Monga, D.; Eliopoulos, G. M.; Moellering, R. C., Jr.; Mylonakis, E. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob. Agents Chemother. 2009, 53, 2605–2609.
Tsai, C. J. Y.; Loh, J. M. S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229.
Zhang, X. X.; Yang, L.; Lu, X. Q.; Lv, Y.; Jiang, D.; Yu, Y.; Peng, Z.; Dong, Z. H. Characterization and property of dual-functional Zn-incorporated TiO2 micro-arc oxidation coatings: the influence of current density. J. Alloys Compd. 2019, 810, 151893.
Ren, L.; Chen, J. X.; Lu, Q.; Wang, C. B.; Han, J.; Huang, K.; Pan, X. N.; Wu, H. Construction of high selectivity and antifouling nanofiltration membrane via incorporating macrocyclic molecules into active layer. J. Membr. Sci. 2020, 597, 117641.
Brogden, K. A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria. Nat. Rev. Microbiol. 2005, 3, 238–250.
Takahashi, D.; Shukla, S. K.; Prakash, O.; Zhang, G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 2010, 92, 1236–1241.
Ganewatta, M. S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29.
Bechinger, B.; Gorr, S. U. Antimicrobial peptides: mechanisms of action and resistance. J. Dent. Res. 2017, 96, 254–260.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51973212) and Department of Science and Technology of Jilin Province (No. 20210203119SF and 20210203173SF).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Notes
The authors declare no competing financial interest.
Electronic Supplementary Information
Rights and permissions
About this article
Cite this article
Liu, H., Liu, X., Cao, YQ. et al. Engineering Antibacterial Activities and Biocompatibility of Hyperbranched Lysine-based Random Copolymers. Chin J Polym Sci 41, 345–355 (2023). https://doi.org/10.1007/s10118-022-2859-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-022-2859-1