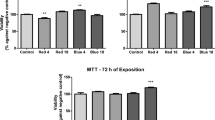
Abstract
Photobiomodulation therapy (PBMT) has been widely used for the promotion of tissue repair. Despite these therapeutic benefits, in some cases, PBMT appears to be unsuccessful, and the strongest hypothesis is that this failure is due to inadequate light dosimetry and wavelengths. The objective of the present critical review was to evaluate the effects of PBMT on cultured keratinocytes using blue, red, or near-infrared light categorized into arbitrary ranges of energy density (0.1–5.0, 5.1–10.0, 10.1–15.0, and over 15.0 J/cm2). The electronic searches were conducted in PubMed, Web of Science, Scopus and LILACS databases, and included LASER or LED devices. A total of 55 articles evaluating the effects of PBMT on cell viability, proliferation, migration, and cytokine and growth factor production were included. Overall, the studies failed to provide detailed information about light dosimetry or detailed experimental conditions. The vast majority of the energy densities tested produced unmodified results regardless of the wavelength applied. However, it was possible to observe that red and near-infrared light had more stimulatory effects than blue light. In addition, for all parameters analyzed, favorable outcomes were mostly obtained in the range of 0.1–5.0 J/cm2. The less explored energy densities were within the 10.1–15.0 J/cm2 range. Energy densities above 15.0 J/cm2 were ineffective or tended to cause cell death. The heterogeneity of the data does not allow us to define a PBMT range setting protocol that would have beneficial effects on keratinocytes.



Similar content being viewed by others
References
Diniz IMA, Carreira ACO, Sipert CR, Uehara CM, Moreira MSN, Freire L, Pelissari C, Kossugue PM, de Araújo DR, Sogayar MC, Marques MM (2018) Photobiomodulation of mesenchymal stem cells encapsulated in an injectable rhBMP4-loaded hydrogel directs hard tissue bioengineering. J Cell Physiol 233:4907–4918. https://doi.org/10.1002/jcp.26309
Elad S, Arany P, Bensadoun RJ, Epstein JB, Barasch A, Raber-Durlacher J (2018) Photobiomodulation therapy in the management of oral mucositis: search for the optimal clinical treatment parameters. Support Care Cancer 26:1–3. https://doi.org/10.1007/s00520-018-4262-6
Engel KW, Khan I, Arany PR (2016) Cell lineage responses to photobiomodulation therapy. J Biophotonics 9:1148–1156. https://doi.org/10.1002/jbio.201600025
Anders JJ, Lanzafame RJ, Arany PR (2015) Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg 33:183–184. https://doi.org/10.1089/pho.2015.9848
Passarella S, Karu T (2014) Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B 140:344–358. https://doi.org/10.1016/j.jphotobiol.2014.07.021
Arany PR, Cho A, Hunt TD, Sidhu G, Shin K, Hahm E, Huang GX, Weaver J, Chen AC, Padwa BL, Hamblin MR, Barcellos-Hoff MH, Kulkarni AB, J Mooney D (2014) Photoactivation of endogenous latent transforming growth factor–β1 directs dental stem cell differentiation for regeneration. Sci Transl Med 6:238ra69. https://doi.org/10.1126/scitranslmed.3008234
George S, Hamblin MR, Abrahamse H (2018) Effect of red light and near infrared laser on the generation of reactive oxygen species in primary dermal fibroblasts. J Photochem Photobiol B 188:60–68. https://doi.org/10.1016/j.jphotobiol.2018.09.004
Ferreira LS, Diniz IMA, Maranduba CMS, Miyagi SPH, Rodrigues MFSD, Moura-Netto C, Marques MM (2018) Short-term evaluation of photobiomodulation therapy on the proliferation and undifferentiated status of dental pulp stem cells. Lasers Med Sci 34(4):659–666. https://doi.org/10.1007/s10103-018-2637-z
Kim JE, Woo YJ, Sohn KM, Jeong KH, Kang H (2017) Wnt/β-catenin and ERK pathway activation: a possible mechanism of photobiomodulation therapy with light-emitting diodes that regulate the proliferation of human outer root sheath cells. Lasers Surg Med 49:940–947. https://doi.org/10.1002/lsm.22736
Yin K, Zhu R, Wang S, Zhao RC (2017) Low-level laser effect on proliferation, migration, and antiapoptosis of mesenchymal stem cells. Stem Cells Dev 26:762–775. https://doi.org/10.1089/scd.2016.0332
Karu TI (2010) Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62:607–610. https://doi.org/10.1002/iub.359
Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94:199–212. https://doi.org/10.1111/php.12864
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4:337–361. https://doi.org/10.3934/biophy.2017.3.337
Mainster MA (2006) Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol 90:784–792. https://doi.org/10.1136/bjo.2005.086553
Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR (2017) Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci Rep 7:7781. https://doi.org/10.1038/s41598-017-07525-w
da Silva JP, da Silva MA, Almeida APF, Junior IL, Matos AP (2010) Laser therapy in the tissue repair process: a literature review. Photomed Laser Surg 28:17–21. https://doi.org/10.1089/pho.2008.2372
Basso FG, Soares DG, Pansani TN, Cardoso LM, Scheffel DL, de Souza Costa CA, Hebling J (2016) Proliferation, migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge. Lasers Surg Med 48:1006–1014. https://doi.org/10.1002/lsm.22553
Houreld NN, Ayuk SM, Abrahamse H (2014) Expression of genes in normal fibroblast cells (WS1) in response to irradiation at 660 nm. J Photochem Photobiol B 130:146–152. https://doi.org/10.1016/j.jphotobiol.2013.11.018
Rizzi M, Migliario M, Tonello S, Rocchetti V, Renò F (2018) Photobiomodulation induces in vitro re-epithelialization via nitric oxide production. Lasers Med Sci 33:1–6. https://doi.org/10.1007/s10103-018-2443-7
Solmaz H, Ulgen Y, Gulsoy M (2017) Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med Sci 32:903–910. https://doi.org/10.1007/s10103-017-2191-0
Tata DB, Waynant RW (2011) Laser therapy: a review of its mechanism of action and potential medical applications. Laser Photonics Rev 5:1–12. https://doi.org/10.1002/lpor.200900032
AlGhamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27:237–249. https://doi.org/10.1007/s10103-011-0885-2
Marques MM, de Cara SPHM, Abe GL, Pedroni ACF, Diniz IMA, Moreira MS (2017) Effects of photobiomodulation therapy in dentoalveolar-derived mesenchymal stem cells: a review of literature. Laser Dent Sci 1:1–7. https://doi.org/10.1007/s41547-017-0002-3
Peplow PV, Chung TY, Baxter GD (2010) Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg Suppl 1:S3–S40. https://doi.org/10.1089/pho.2010.2771
World Association of Laser Therapy (WALT) (2006) Consensus agreement on the design and conduct of clinical studies with low-level laser therapy and light therapy for musculoskeletal pain and disorders. Photomed Laser Surg 24:761–762. https://doi.org/10.1089/pho.2006.24.761
Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA (2014) Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122:711–718. https://doi.org/10.1289/ehp.1307972
Basso FG, Oliveira CF, Kurachi C, Hebling J, de Souza Costa CA (2013) Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med Sci 28:367–374. https://doi.org/10.1007/s10103-012-1057-8
Sperandio FF, Simões A, Corrêa L, Aranha AC, Giudice FS, Hamblin MR, Sousa SC (2015) Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J Biophotonics 8:795–803. https://doi.org/10.1002/jbio.201400064
Castellano-Pellicena I, Uzunbajakava NE, Mignon C, Raafs B, Botchkarev VA, Thornton MJ (2018) Does blue light restore human epidermal barrier function via activation of opsin during cutaneous wound healing? Lasers Surg Med 51(4):370–382. https://doi.org/10.1002/lsm.23015
Walter C, Pabst AM, Ziebart T (2015) Effects of a low-level diode laser on oral keratinocytes, oral fibroblasts, endothelial cells and osteoblasts incubated with bisphosphonates: an in vitro study. Biomed Rep 3:14–18. https://doi.org/10.3892/br.2014.389
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8:579–591. https://doi.org/10.1038/nrd2803
Gagnon D, Gibson TW, Singh A, zur Linden AR, Kazienko JE, LaMarre J (2016) An in vitro method to test the safety and efficacy of low-level laser therapy (LLLT) in the healing of a canine skin model. BMC Vet Res 12:73. https://doi.org/10.1186/s12917-016-0689-5
Lee JB, Bae SH, Moon KR, Na EY, Yun SJ, Lee SC (2016) Light-emitting diodes downregulate cathelicidin, kallikrein and toll-like receptor 2 expressions in keratinocytes and rosacea-like mouse skin. Exp Dermatol 25:956–961. https://doi.org/10.1111/exd.13133
Liebmann J, Born M, Kolb-Bachofen V (2010) Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Invest Dermatol 130:259–269. https://doi.org/10.1038/jid.2009.194
Migliario M, Rizzi M, Rocchetti V, Cannas M, Renò F (2013) In vitro toxicity of photodynamic antimicrobial chemotherapy on human keratinocytes proliferation. Lasers Med Sci 28:565–569. https://doi.org/10.1007/s10103-012-1112-5
Szeimies RM, Abels C, Fritsch C, Karrer S, Steinbach P, Bäumler W, Goerz G, Goetz AE, Landthaler M (1995) Wavelength dependency of photodynamic effects after sensitization with 5-aminolevulinic acid in vitro and in vivo. J Invest Dermatol 105:672–677. https://doi.org/10.1111/1523-1747.ep12324377
Opal SM, Depalo V (2000) Anti-inflammatory cytokines. Chest 117:1162–1172. https://doi.org/10.1378/chest.117.4.1162
Fushimi T, Inui S, Nakajima T, Ogasawara M, Hosokawa K, Itami S (2012) Green light emitting diodes accelerate wound healing: characterization of the effect and its molecular basis in vitro and in vivo. Wound Repair Regen 20:226–235. https://doi.org/10.1111/j.1524-475X.2012.00771.x
Haas AF, Wong JW, Iwahashi CK, Halliwell B, Cross CE, Davis PA (1998) Redox regulation of wound healing? NF-kappaB activation in cultured human keratinocytes upon wounding and the effect of low energy HeNe irradiation. Free Radic Biol Med 25:998–1005. https://doi.org/10.1016/S0891-5849(98)00135-X
Yu HS, Chang KL, Yu CL, Chen JW, Chen GS (1996) Low-energy helium-neon laser irradiation stimulates interleukin-1 alpha and interleukin-8 release from cultured human keratinocytes. J Invest Dermatol 107:593–596. https://doi.org/10.1111/1523-1747.ep12583090
Feliciani C, Gupta AK, Saucier DN (1996) Keratinocytes and cytokine/growth factors. Crit Rev Oral Biol Med 7:300–318
Baroni A, De Filippis A, Oliviero G, Fusco A, Perfetto B, Buommino E, Donnarumma G (2017) Effect of 1064-nm Q-switched Nd: YAG laser on invasiveness and innate immune response in keratinocytes infected with Candida albicans. Lasers Med Sci 33:941–948. https://doi.org/10.1007/s10103-017-2407-3
Lim W, Kim J, Lim C, Kim S, Jeon S, Karna S, Cho M, Choi H, Kim O (2012) Effect of 635 nm light-emitting diode irradiation on intracellular superoxide anion scavenging independent of the cellular enzymatic antioxidant system. Photomed Laser Surg 30:451–459. https://doi.org/10.1089/pho.2011.3199
Mignon C, Uzunbajakava NE, Raafs B, Botchkareva NV, Tobin DJ (2017) Photobiomodulation of human dermal fibroblasts in vitro: decisive role of cell culture conditions and treatment protocols on experimental outcome. Sci Rep 7:2797. https://doi.org/10.1038/s41598-017-02802-0
Tucker LD, Lu Y, Dong Y, Yang L, Li Y, Zhao N, Zhang Q (2018) Photobiomodulation therapy attenuates hypoxic-ischemic injury in a neonatal rat model. J Mol Neurosci 65:514–526. https://doi.org/10.1007/s12031-018-1121-3
Acknowledgments
Mrs. E. Greene provided English editing of the manuscript.
Funding
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). T.A.S. and R.A.M. are CNPq research fellows. P.T.R.A. and J.A.A.A. are the recipients of fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval is not applicable in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
(DOCX 32.5 kb)
Supplementary Table 2
(DOCX 35.3 kb)
Supplementary Table 3
(DOCX 57.8 kb)
Supplementary Table 4
(DOCX 39.3 kb)
Supplementary Table 5
(DOCX 31.9 kb)
Supplementary References 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
de Abreu, P.T.R., de Arruda, J.A.A., Mesquita, R.A. et al. Photobiomodulation effects on keratinocytes cultured in vitro: a critical review. Lasers Med Sci 34, 1725–1734 (2019). https://doi.org/10.1007/s10103-019-02813-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02813-5