Abstract
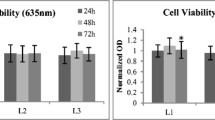
Photobiomodulation (PBM) and photodynamic therapy (PDT) share similar mechanisms but have opposite aims. Increased levels of reactive oxygen species (ROS) in the target tissue in response to light combined photosensitizer (PS) application may lead to cell proliferation or oxidative damage depending on the ROS amount. The purpose of the present study is to investigate the effect of indocyanine green (ICG)-mediated PBM on osteoblast cells by measuring cell viability, proliferation, alkaline phosphatase (ALP) activity, mineralization, and gene expressions of three phenotypic osteoblast markers. A diode laser irradiating at 809 nm (10 W output power, 50 mW/cm2 power density) was used at 0.5, 1, and 2 J/cm2 energy densities (10, 20, and 40 s respectively) was applied following ICG incubation. No inhibitory effect was observed in cell viability and proliferation according to the (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and Alamar Blue assays. ICG-mediated PBM did not alter cell viability but increased ALP activity and enhanced mineralization of existing osteoblasts. These results were also confirmed by real-time polymerase chain reaction (RT-PCR) analysis of osteoblastic markers. PS can be combined to PBM not only to damage the malignant cells as aimed in PDT studies, but also to promote cellular activity. The findings of this in vitro study may contribute to in vivo studies and ICG-mediated PBM can have promising outcomes in bone healing and regeneration therapies in future.






Similar content being viewed by others
References
Kim W-S, Calderhead RG (2011) Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Ther 20:205–215. https://doi.org/10.5978/islsm.20.205
Kushibiki T, Tajiri T, Ninomiya Y, Awazu K (2010) Chondrogenic mRNA expression in prechondrogenic cells after blue laser irradiation. J Photochem Photobiol B 98:211–215. https://doi.org/10.1016/j.jphotobiol.2010.01.008
Liebert MA (2006) Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg 24:705–714. https://doi.org/10.1089/PHO.2006.1076
Hu W-P, Wang J-J, Yu C-L et al (2017) Helium–Neon Laser Irradiation Stimulates Cell Proliferation through Photostimulatory Effects in Mitochondria. J Invest Dermatol 127:2048–2057. https://doi.org/10.1038/sj.jid.5700826
AlGhamdi KM, Kumar A, N a M (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27:237–249. https://doi.org/10.1007/s10103-011-0885-2
Chen AC-H, Huang Y-Y, Arany PR, Hamblin MR (2009) <title>Role of reactive oxygen species in low level light therapy</title>. 716502-716502–11. doi: https://doi.org/10.1117/12.814890
Droge W, Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95. https://doi.org/10.1152/physrev.00018.2001
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255. https://doi.org/10.1038/sj.bjp.0705776
DeRosa MC, Crutchley RJ (2002) Photosensitized singlet oxygen and its applications. Coord Chem Rev 233–234:351–371. https://doi.org/10.1016/S0010-8545(02)00034-6
FOX IJ, WOOD EH (1960) Indocyanine green: physical and physiologic properties. Proc Staff Meet Mayo Clin 35:732–744
Desmettre T, Devoisselle JM, Mordon S (2000) MAJOR REVIEW Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. 45
Yusa K, Yamamoto O, Iino M et al (2016) Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch Oral Biol 71:162–169. https://doi.org/10.1016/j.archoralbio.2016.07.010
Coombe AR, Ho CT, Darendeliler MA et al (2001) The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res 4:3–14
Ebrahimi T, Moslemi N, Rokn A et al (2012) The influence of low-intensity laser therapy on bone healing. J Dent (Tehran) 9:238–248
Arisu HD, Türköz E, Bala O (2006) Effects of Nd:Yag laser irradiation on osteoblast cell cultures. Lasers Med Sci 21:175–180. https://doi.org/10.1007/s10103-006-0398-6
Bolukbasi Ates G, Ak Can A, Gulsoy M (2017) Investigation of photobiomodulation potentiality by 635 and 809 nm lasers on human osteoblasts. Lasers Med Sci 32:591–599. https://doi.org/10.1007/s10103-017-2153-6
Cavalcanti MFXB, Maria DA, De Isla N, et al (2015) Evaluation of the proliferative effects induced by low-level laser therapy in bone marrow stem cell culture. Photomed Laser Surg doi: https://doi.org/10.1089/pho.2014.3864
Borzabadi-Farahani A (2016) Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J Photochem Photobiol B Biol 162:577–582. https://doi.org/10.1016/j.jphotobiol.2016.07.022
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Pettit RK, Weber CA, Kean MJ et al (2005) Microplate alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chemother 49:2612–2617. https://doi.org/10.1128/AAC.49.7.2612-2617.2005
Rampersad SN (2012) Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Switzerland) 12:12347–12360. https://doi.org/10.3390/s120912347
Zachari MA, Chondrou PS, Pouliliou SE et al (2014) Evaluation of the alamarblue assay for adherent cell irradiation experiments. Dose-Response 12:246–258. https://doi.org/10.2203/dose-response.13-024.Koukourakis
Gregory CA, Gunn WG, Peister A, Prockop DJ (2004) An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 329:77–84. https://doi.org/10.1016/j.ab.2004.02.002
Ateş GB, Ak A, Garipcan B, Gülsoy M (2017) Methylene blue mediated photobiomodulation on human osteoblast cells. Lasers Med Sci 32:1847–1855. https://doi.org/10.1007/s10103-017-2286-7
Arany PR (2011) Laser photobiomodulation: models and mechanisms CU R R ENT
Schwartz-Filho HO, Reimer AC, Marcantonio C et al (2011) Effects of low-level laser therapy (685 nm) at different doses in osteogenic cell cultures. Lasers Med Sci 26:539–543. https://doi.org/10.1007/s10103-011-0902-5
Huang Y-Y, Chen AC-H, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose Response 7:358–383. https://doi.org/10.2203/dose-response.09-027.Hamblin
Neupane J, Ghimire S, Shakya S et al (2010) Effect of light emitting diodes in the photodynamic therapy of rheumatoid arthritis. Photodiagn Photodyn Ther 7:44–49. https://doi.org/10.1016/j.pdpdt.2009.12.006
Montoro LA, Turrioni APS, Basso FG et al (2014) Infrared LED irradiation photobiomodulation of oxidative stress in human dental pulp cells. Int Endod J 47:747–755. https://doi.org/10.1111/iej.12211
Rezai KA, Farrokh-Siar L, Ernest JT, van Seventer GA (2004) Indocyanine green induces apoptosis in human retinal pigment epithelial cells. Am J Ophthalmol 137:931–933. https://doi.org/10.1016/j.ajo.2003.11.016
Yamada T, Yoshikawa M, Kanda S et al (2002) In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells 20:146–154. https://doi.org/10.1634/stemcells.20-2-146
Abels C, Fickweiler S, Weiderer P et al (2000) Indocyanine green (ICG) and laser irradiation induce photooxidation. Arch Dermatol Res 292:404–411. https://doi.org/10.1007/s004030000147
Bäumler W, Abels C, Karrer S et al (1999) Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br J Cancer 80:360–363. https://doi.org/10.1038/sj.bjc.6690363
Jawad MM, Husein A, Azlina A et al (2013) Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J Biomed Opt 18:128001. https://doi.org/10.1117/1.JBO.18.12.128001
Amid R, Kadkhodazadeh M, Ahsaie MG, Hakakzadeh A (2014) Effect of low level laser therapy on proliferation and differentiation of the cells contributing in bone regeneration. J Lasers Med Sci 5:163–170
Pagin MT, de Oliveira FA, Oliveira RC et al (2014) Laser and light-emitting diode effects on pre-osteoblast growth and differentiation. Lasers Med Sci 29:55–59. https://doi.org/10.1007/s10103-012-1238-5
Zancanela DC, Primo FL, Rosa AL et al (2011) The effect of photosensitizer drugs and light stimulation on osteoblast growth. Photomed Laser Surg 29:699–705. https://doi.org/10.1089/pho.2010.2929
Medina-Huertas R, Manzano-Moreno FJ, De Luna-Bertos E et al (2014) The effects of low-level diode laser irradiation on differentiation, antigenic profile, and phagocytic capacity of osteoblast-like cells (MG-63). Lasers Med Sci. https://doi.org/10.1007/s10103-014-1557-9
Petri AD, Teixeira LN, Crippa GE et al (2010) Effects of low-level laser therapy on human osteoblastic cells grown on titanium. Braz Dent J 21:491–498
Stein E, Koehn J, Sutter W et al (2008) Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien Klin Wochenschr 120:112–117. https://doi.org/10.1007/s00508-008-0932-6
Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K (2005) Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 26:3503–3509. https://doi.org/10.1016/j.biomaterials.2004.09.033
Rodan GA, Noda M (1991) Gene expression in osteoblastic cells. Crit Rev Eukaryot Gene Expr 1:85–98
Golub EE, Boesze-Battaglia K (2007) The role of alkaline phosphatase in mineralization. Curr Opin Orthop 18:444–448. https://doi.org/10.1097/BCO.0b013e3282630851
Setzer B, Bächle M, Metzger MC, Kohal RJ (2009) The gene-expression and phenotypic response of hFOB 1.19 osteoblasts to surface-modified titanium and zirconia. Biomaterials 30:979–990. https://doi.org/10.1016/j.biomaterials.2008.10.054
Huang W, Yang S, Shao J, Li Y-P (2007) Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci 12:3068–3092
Silva JCE, Lacava ZGM, Kuckelhaus S et al (2004) Evaluation of the use of low level laser and photosensitizer drugs in healing. Lasers Surg Med 34:451–457. https://doi.org/10.1002/lsm.20062
Jayasree RS, Gupta AK, Rathinam K et al (2001) The influence of photodynamic therapy on the wound healing process in rats. J Biomater Appl 15:176–186. https://doi.org/10.1106/9335-Q0NC-5XCQ-KBYK
Zhang X, Jiang F, Zhang ZG et al (2005) Low-dose photodynamic therapy increases endothelial cell proliferation and VEGF expression in nude mice brain. Lasers Med Sci 20:74–79. https://doi.org/10.1007/s10103-005-0348-8
Funding
This study was supported by grant (113Z059) of the Scientific and Research Council of Turkey (TUBITAK). The cell culture experiments were performed at Boğaziçi University Biomedical Engineering Institute Biomaterials laboratory, which was supported by Boğaziçi University Research Fund with the grant number 6701.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ateş, G.B., Ak, A., Garipcan, B. et al. Indocyanine green-mediated photobiomodulation on human osteoblast cells. Lasers Med Sci 33, 1591–1599 (2018). https://doi.org/10.1007/s10103-018-2530-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2530-9