Abstract
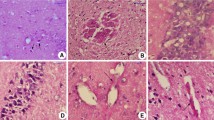
We tested whether low-dose photodynamic therapy (PDT) induces an angiogenic response in the normal brain of nude mice (n=20). Normal brains of nude mice were subjected to PDT at low doses (Photofrin: 2 mg/kg; optical: 2 J/cm2 and 4 J/cm2). BrdU (50 mg/kg) was injected (intraperitoneally, i.p.) daily from PDT treatment to sacrifice (1 and 2 weeks after PDT). Laser scanning confocal microscopy, immunohistochemistry, and immunofluorescence staining were performed to assay angiogenic response. Morphological results show no significant tissue damage induced by PDT and two- and three-dimensional image analyses revealed no significant difference in vascular structure between the areas of exposure to PDT and contralateral areas in all mice. However, the number of BrdU immunoreactive cells were significantly increased in the areas of PDT treatment compared with contralateral hemisphere in both groups, and the number of BrdU-positive cells increased in a PDT-dose-dependent manner. Furthermore, immunohistochemical data indicate that PDT at these low doses significantly induces the expression of the vascular endothelial growth factor (VEGF) in PDT-treated regions in the 1-week group, but not in the 2-week group. These data indicate that low-dose PDT results in increased VEGF expression and endothelial cell proliferation in normal brains.






Similar content being viewed by others
References
Nicholas MK, Prados MD, Larson DA (1997) Malignant astrocytomas. In: Black PM, Loeffler J (eds) Cancer of the nervous system. Blackwell Publishers, Oxford, UK, pp 464–491
Brem S, Cotran R, Folkman J (1972) Tumor angiogenesis: a quantitative method for histologic grading. J Natl Cancer Inst 48:347–356
Henderson BW, Dougherty TJ (1992) How does photodynamic therapy work? Photochem Photobiol 55:145–157
Muller P, Wilson B (1991) Photodynamic therapy of brain tumours—post-operative “field fractionation.” J Photochem Photobiol B 9:117–125
Kay AH, Hill JS (1993) Photodynamic therapy of brain tumours. Ann Acad Med Singapore 22:470–481
Kostron H, Plangger C, Fritsch E, Maier H (1990) Photodynamic treatment of malignant brain tumors. Wien Klin Wochenschr 102:531–535
Jiang F, Lilge L, Grenier J, Li Y, Wilson MD, Chopp M (1998) Photodynamic therapy of U87 human glioma in nude rat using liposome-delivered photofrin. Lasers Surg Med 22(2):74–80
Jiang F, Lilge L, Logie B, Li Y, Chopp M (1997) Photodynamic therapy of 9L gliosarcoma with liposome-delivered photofrin. Photochem Photobiol 65(4):701–706
Chopp M, Dereski MO, Madigan L, Jiang F, Logie B (1996) Sensitivity of 9L gliosarcomas to photodynamic therapy. Radiat Res 146(4):461–465
Chopp M, Madigan L, Dereski M, Jiang F, Li Y (1996) Photodynamic therapy of human glioma (U87) in the nude rat. Photochem Photobiol 64(4):707–711
Jiang F, Zhang ZG, Katakowski M, Robin AM, Faber M, Zhang F, Chopp M (2004) Angiogenesis induced by photodynamic therapy in normal rat brains. J Photochem Photobiol 79(6):494–498
del Zoppo GJ (1994) Microvascular changes during cerebral ischemia and reperfusion. Cerebrovasc Brain Metab Rev 6:47–96
Shahrokni A, Zoroofi RA, Soltanian-Zadeh H (2001) Fast skeletonization algorithm for 3-D elongated objects. In: Proceedings of SPIE Medical Imaging 2001 image processing conference, San Diego, California, 17–22 February 2001. International Society for Optical Engineering, Bellingham, Washington, pp 323–330
Zhang ZG, Chopp M, Goussev A, Lu D, Morris D, Tsang W, Powers C, Ho KL (1999) Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats. J Neurosci 19:10898–10907
Li Y, Jiang N, Powers C, Chopp M (1998) Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke 29(9):1972–1980; Discussion 1980–1981
Dereski MO, Chopp M, Garcia JH, Hetzel FW (1991) Depth measurements and histopathological characterization of photodynamic therapy generated normal brain necrosis as a function of incident optical energy dose. Photochem Photobiol 54(1):109–112
Chen Q, Chopp M, Dereski MO, Wilson BC, Patterson MS, Schreiber A, Hetzel FW (1992) The effect of light fluence rate in photodynamic therapy of normal rat brain. Radiat Res 132(1):120–123
Nichols MG, Foster TH (1994) Oxygen diffusion and reaction kinetics in the photodynamic therapy of multicell tumour spheroids. Phys Med Biol 39(12):2161–2181
Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M (2003) Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res 92:308–313
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Jiang, F., Zhang, Z. et al. Low-dose photodynamic therapy increases endothelial cell proliferation and VEGF expression in nude mice brain. Lasers Med Sci 20, 74–79 (2005). https://doi.org/10.1007/s10103-005-0348-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-005-0348-8