Abstract
The quality and properties of biochar are generally influenced by the nature of the raw materials and pyrolysis techniques. To assess the quality of sesame biochar production, a disc chamber reactor set on a solar parabolic dish concentrator was proposed as a modified slow pyrolysis technique. To evaluate the physicochemical characterizations of the produced biochar, two pyrolysis settings were used: 470 °C for 1 h (T1) and 440 °C for 2 h (T2) to produce biochar from sesame stalk feedstock (SS) using the proposed solar disk chamber reactor. Ash content, mass fraction of elements (C, H, and O%), pH, surface area, zeta potential, Fourier transform infrared (FTIR), and scanning electron microscope (SEM) were investigated. The results showed that the mass of T1 biochar decreased by 5% when compared to T2, while ash content, pH, fixed carbon, and volatile gases for both biochars were relatively close. The H/C and O/C molar ratios were below 1.00 and 0.4, respectively, indicating a loss of degradable polar contents and the formation of aromatic compounds. The surface area of T2 biochar was three times the surface area of T1, with the opposite trend in mean pore diameter. Two biochars showed the same FTIR peaks and SEM data, with small differences in their characteristics, demonstrating that pyrolysis time and temperature had a tight relationship. Both biochars showed approximately similar properties. The reactor’s efficiency is mainly affected by solar energy and atmospheric conditions during operation, which influence the average surface temperature. In Egypt, climatic conditions would be more favorable in the summer to improve the efficiency of parabolic solar dish concentrators for producing high-quality biochar.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biochar is a recalcitrant carbon-rich compound derived from organic biomass through the thermochemical decomposition process in an oxygen-controlled environment. The process mentioned to create biochar is called pyrolysis (Lehmann et al. 2011). Biochar has a high-stability carbon structure due to its high content of condensed aromatic structures. Additionally, it has distinctive properties like a high content of organic carbon, high porosity, a large surface area, various and abundant surface functional groups, an alkaline pH, diverse elemental composition (Ca, Mg, K, Na, P, Cu, Zn, Fe, etc.), and a high cation exchange capacity (Tomczyk et al. 2020). Thus, biochar has gained considerable attention in numerous environmental fields, including soil amendments, the elimination of organic and inorganic pollutants from wastewater or polluted soil, mitigating greenhouse gas emissions, and sequestering carbon emissions (Lehmann et al. 2011). The quality and characterization of biochar are generally affected by the nature of the raw materials and pyrolysis techniques.
In Egypt, the amount of bioenergy crop residue is approximately 12.33 × 106 tons/year (2000–2016) as dry biomass, including maize, rice straw, cotton, sugar beet, sesame, lupine, sorghum, and peanut residues (Said et al. 2013). Sesame is considered to be one of the oldest oilseed crops in Egypt since the Ptolemaic period (Shaw 2003). Its average cultivation area was 73.73 thousand feddans (2000–2018), with an average production of 3928.90 thousand tons (Fangary 2021). Annually, the average production capacity of sesame is 94.47 thousand tons (Elbadrashini et al. 2019). Generally, bioenergy crop residues are not palatable for animals feeding before enhancing their nutritional value through physical, chemical, and biological pretreatments to improve digestion rates and palatability (Elbadrashini et al. 2019).
Thermochemical processes like pyrolysis and torrefaction have been applied to sesame stalks to improve their physicochemical characteristics. These could be used as valuable materials in wastewater treatments, as sorbent materials for pollutant removal, and as agricultural soil enhancers. It is confirmed that the highest temperature and residence time of the torrefaction method for sesame stalk biochar production (generated from 200 to 300 °C for 30 and 60 min) produced the highest carbon mass fraction (55.77%) and fixed carbon (55.10%). On the other hand, biochar yield, oxygen content, and hydrogen content declined to their lowest values with increasing temperatures, resulting in decreased H/C and O/C molar ratios due to the decay of hemicellulosic and cellulosic compounds (Khairy et al. 2023). Park et al. (2015) detected a similar observation by studying the effect of pyrolysis temperature on the sesame biochar characteristics and its phosphorus release. They observed that the functional groups of the sesame biochar changed due to the raised pyrolysis temperature, which led to the disappearance of aliphatic C–H stretching bonds, a decrease in the peaks of C=O stretching vibrations, and an instance of secondary aromatic amines. However, sesame straw biochar has a significant amount of nutrients, which increase with increasing pyrolysis temperatures as the ash content increases. Park et al. (2015) reported that the release of phosphorus from sesame biochar straw was significantly affected by pyrolysis temperature, which increased 2.7 times due to the raised pyrolysis temperature (from 300 to 600 °C).
The pyrolysis techniques are mainly classified into slow and fast pyrolysis to produce biochar, biogas, and bio-oil based on pyrolysis operating systems like residence time, temperature, and heating rate (Xie et al. 2015; Zaman et al. 2017).
Fast pyrolysis can produce, on average, 70% (wt) of gas (biofuel) and a low amount of lightweight and black residue (biochar). The operating conditions of this process are high temperatures with an accelerated heating rate. In contrast, the slow pyrolysis process of lignocellulosic materials can create high-quality biochar up to 30–35% from dry raw-material weight, which operates at low temperatures and low heating rates (Zaman et al. 2017; Tomczyk et al. 2020).
In slow pyrolysis, the typical source of heating energy in the pyrolysis chamber is an external source that may be provided from electrical energy sources, burning of the syngas formed during the thermal decomposition (self-sustaining), or external direct burning of the biomass feedstock (Ronsse et al. 2013). The common pyrolysis reactors for biochar production are fixed bed reactors, batch reactors, semi-reactors, plasma reactors, rotary kilns, auger reactors, fluidized bed reactors, microwave-assisted reactors, traditional reactors, and solar reactors (Zaman et al. 2017; El-Gamal et al. 2017; Jouhara et al. 2018; Lewandowski et al. 2019). A solar radiation collector is a unique option for producing biofuel and biochar due to its renewable and costless nature (Chintala et al. 2017).
Recently, numerous solar collector reactors have been used to produce bio-oil and biochar by concentrating the radiation beam directly into the solar pyrolysis chamber, including ellipsoidal concentrators, deep-dish parabolic mirrors, Fresnel lenses, mirrors, parabolic dish collectors, and hyperboloid concentrators (Rony et al. 2018). The pyrolysis temperature of these reactors ranged from 300 to 900 °C (Ndukwu et al. 2021). The main advantage of using solar energy in the pyrolysis process is that provides a clean way to eliminate the CO2 emissions that were produced when using the direct firing of the organic waste. Biochar produced at low pyrolysis temperatures (300 °C and 400 °C) was shown to be the most effective in promoting various crop growth when applied with mineral fertilizer, enhancing soil water retention, see Laghari et al. (2015).
The total area of Egypt, which is located in the semi-arid region, is 1 million km2 and most of this area is non-inhabited deserts that occupy about 96% of the total area (Ebaid et al. 2016). According to the Climate Change Knowledge Portal report, the current climatology in Egypt, 1991–2021, is hot and dry in the summer (37.5 ℃ on average, from June to August) and warm in the winter with an average rainfall of 50 mm/year. Egypt has a large amount of solar radiation as a source of renewable energy. Khalil et al. (2010) reported that the duration of sunshine throughout the year ranges from 9 to 11 h with a few cloudy days. This energy demonstrates the high economic potential for power generation (about 74,000 TWh/year).
Because of its great capacity to focus solar radiation on a specific spot, the parabolic dish concentrator proposed in our earlier work (El-Gamal et al. 2022) is the most preferred. The solar parabolic dish collector was used to pyrolyze sesame stalk residues at 300 ℃ for 3 h. The authors noted that the formed biochar had heterogeneous physicochemical properties compared to those formed by the conventional (muffle furnace) method under the same conditions because the temperature distribution was not uniform in the solar reactor. Therefore, the main novelty of the present study is to present a new design of the solar reactor chamber that can guarantee uniform temperature distribution with higher temperature levels to improve the resulting physicochemical properties of the produced biochar. The new design is smaller in volume, which enables much more heat flux to be concentrated on the reactor, which in turn allows for more samples and various residence times of the sample to be tested. The sesame feedstock was used to evaluate the pyrolysis conditions of the produced biochar at different ranges of temperatures and residence times to investigate the most suitable application of the produced biochar. Moreover, the simplicity of the new design allows the repetition of the same tests on various samples of agricultural wastes.
Materials and methods
Modified solar disk chamber design
The modified design of the parabolic solar dish reactor tested by El-Gamal et al. (2022) resulted in diverse physicochemical properties of the produced biochar due to its large size and non-uniform temperature distribution along the chamber side walls. Therefore, the modified design is proposed to be smaller in diameter and slimmer in height to allow for more heat flux, minimize the heat losses through the sides, and attain higher temperature levels. Figure 1a shows the entire assembly of the reactor chamber installed on the focal point of the parabolic trough solar collector during the field tests. The chamber resembles a disk fabricated of galvanized steel sheets 1 mm thick, with an inner diameter of 17.5 cm and a depth of 1.5 cm (Fig. 1b). It is appropriate to hold around 30 g of agricultural residues. The cover has an outer diameter of 17.5 cm and was designed to shrink with the disk when closing the chamber. The concentrator is designed to reach a target temperature of around 300 °C and is suitable for a temperature of 500 °C during the summer. The chamber was placed inside a conical-shaped cavity, as shown in Fig. 1c. The inner surface of the cavity was covered by glass mirror segments, and the rod that carries the reactor chamber can be adjusted to ensure that the disk is located at the focal spot. The cavity is insulated from the side walls and the top. Three typical chambers were designed to be able to carry out several experiments during the daytime. Although the tracking mechanism of the parabolic trough relies on LDR sensors, it can be switched to manual mode to allow chamber replacement.
Biochar preparation and characterization
The air-dried sesame (Sesamum indicum) stalk residue (5 cm) was chosen to produce biochar. The stalk was placed inside the disk chamber reactor, and the movable cover was well sealed to avoid oxygen and burn the material in an inert environment. The pyrolysis chamber reactor was fixed on a stand facing the solar parabolic dish collector and pyrolyzed for 1 h (T1, time 1) and 2 h (T2, time 2). Pyrolysis temperature has been recorded during the pyrolysis processes using thermocouples of type k. The produced biochars are labeled hereafter as (T1 and T2), in reference to their pyrolysis times of 1 and 2 h, respectively. The temperature of the pyrolytic dish reactor was recorded randomly during the thermal decomposition process, and the average temperature during pyrolysis was about 470 °C and 420 ℃ for T1 and T2 of pyrolysis time, respectively. The formed biochars were kept in the disk reactor until cooled to ambient temperature; the dry weight was recorded, ground, and passed through 2 mm sieve. Some physical and chemical characterizations of the produced biochars were studied to evaluate the modified disk reactor’s temperature distribution during the pyrolysis process. The biochar yield was calculated according to the following Eq. (1).
where BW is the dry weight of the biochar (g), and FW is the air-dried weight of the sesame feedstock (g).
The moisture content (MC, %) of sesame stalk feedstock (SS) and biochar samples (T1 and T2) was determined by calculating the weight loss of dried samples at 80 ℃ for 24 h (Samsuri et al. 2014). The volatile matter content (VM, %) was calculated based on the mass loss after being combusted at 850 °C for 1 min using a muffle furnace (HYSC, Hanyang Science Lab Co., Ltd., Seoul, Korea) according to Sun et al. (2017), while the ash content (AC, %) was determined after dry combustion at 600 °C for 12 h as explained by El-Gamal et al. (2022). Fixed-carbon content (FC, %) was calculated by subtracting the percentages of MC, VM, and AC from the total weight of the air-dried sesame stalk residue (SS) following the Eq. (2):
The equation was used to calculate the FC for the air-dried biochar (T1 and T2). Based on the mathematical correlation suggested by Klasson (2017) to determine the mass fraction of carbon, hydrogen, and oxygen and the mole ratios of H/C and O/C for the biochar, the following equations were applied:
The pH values were measured at ambient temperature for feedstock and biochar suspension (1:100, w:v) after stirring for 20 min at 90 ℃ using a calibrated pH-meter model, Accumet Research AR50 (Masulili et al. 2010).
The BET (Brunauer–Emmett–Teller) analysis technique was used to measure the surface area of the two biochar types and their porosity distribution by using the N2 adsorption–desorption isotherm at 77 K on a gas sorption analyzer (Micro-trac MRB BELSORP-mini X, Japan). The zeta potential and particle size analysis were used to determine the negative charges of the surface of SS, T1, and T2 powders and their particle sizes, respectively, using the powder-water suspension (0.1 g: 200 ml) to be detected by the Zetasizer (ZP-Malvern-UK) after shaking at 150 rpm for 12 h (Hong et al. 2019). Compositional analysis was used to determine the surface functional groups using a Fourier transform infrared (FTIR) spectrophotometer (FT/IR-5300, JASCO Corporation, Japan). The adsorbent spectra of feedstock and biochar mounted on potassium bromide were recorded under the range of 4000–400 cm−1 (Tsai et al. 2021). The characterization of the surface morphology of gold-coated samples was investigated using scanning electron microscopy (SEM, JEOL, Model JSM—IT200, Japan) at various X500 and X2000 magnification scales.
Results and discussion
Solar pyrolysis temperature
In the present tests, it was planned to keep the temperature inside the reactor above 300° for the two typical sesame samples for one and two hours, respectively. The temperature history of both samples is plotted in Fig. 2 with average temperatures of 470 ± 39.7 °C (T1) and 440 ± 11.8 °C (T2). It is worth mentioning that the two samples were pyrolyzed on two different days, which explains the difference between the average temperature values of the two samples. This may affect the reached temperature during the pyrolysis process that can be influenced by variable environmental conditions along the operating time. It is known that the major factors in the pyrolysis process affecting biochar yield and its physicochemical properties are heating rate, temperature, and residence time (Tomczyk et al. 2020). During low pyrolysis temperatures (below 200 °C), the temperature causes the evaporation of moisture and volatilization of light compounds. Evaporation of moisture causes leakage of light bonds and thus the formation of hydroperoxides, –COOH, and –CO groups (Cárdenas-Aguiar et al. 2017). At temperatures in the range of 200–500 ℃, hemicellulose and cellulose could be devolatilized and destroyed, but the formation of strong carbon structures occurred at temperatures above 500 ℃ (Tomczyk et al. 2020).
Sesame stalk feedstock and biochar characterization
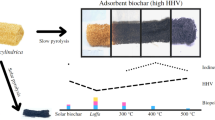
Images of sesame stalk feedstock (SS) and the produced biochar types (T1 and T2) are presented in Fig. 3. SS has a distinct color difference from both biochar. The biochar pyrolyzed at ≃ 470 ℃ for one hour (T1) was light black in color (Fig. 3b), while the biochar pyrolyzed at ≃ 440 ℃ for two hours was uniformly dark black (Fig. 3c). A homogeneous color observed in the produced biochar samples mainly indicating a uniform distribution of solar temperature in the disk reactor. In our previous study, the used solar chamber reactor generated a brown heterogeneous biochar yield with a carbon content ranged from 49 to 53% due to the non-uniform distribution of solar temperature in the reactor field (El-Gamal et al. 2022). In this study, the darker color of the produced biochars, with a carbon content of 54–56%, may indicate that the generated biochars were more carbonized and stabilized, probably due to the homogenous distribution of the solar temperature. This may assume that the new reactor is more efficient because of the high temperature probably because of the design reactor that permits to concentrate and distribute the solar temperature around the new reactor, with a thinner thickness, compared to the old reactor. The recorded high pyrolysis temperatures (300–500 °C) may accelerate the depletion of oxygen and hydrogen elements (Xie et al. 2015).
The effects of pyrolysis temperature and residence time on physicochemical characterizations of yielded biochar types using a solar disk reactor are illustrated in Table 1. The yield of sesame stalk biochar produced at ≃ 470 ℃ for 1 h (T1) was lower than that produced at ≃ 440 ℃ for 2 h (T2) as it decreased by 5%. It can be seen that the pyrolysis temperature has a higher effect on the biochar yield than the residence time. Although the residence time of T2 was longer than that of T1 (Fig. 2), the yield of biochar was less due to the differences in the contribution of solar energy to the pyrolysis process. Sun et al. (2017) concluded that residence time had a slighter impact on the yielded biochar amount than the pyrolysis temperature. It is reported that the yielded biochar of apple tree branches was reduced from 47.94 to 28.48% by increasing the pyrolysis temperature from 300 to 600 ℃ (Zhao et al. 2017). Additionally, El-Gamal et al. (2017) observed that the yielded biochar of sugarcane (SCBB) and rice husk (RHB) pyrolyzed at 450, 500, and 550 ℃ decreased from 41.71 to 34.93% for SCBB and from 46.17 to 39.92% for RHB. Thus, the yield of biochar is intensely associated with changes in pyrolysis temperatures. Tomczyk et al. (2020) confirmed that at low temperatures (≤ 500 ℃), most of the easily degradable compounds (cellulosic and hemicellulosic compounds) and unstable fractions (volatile matter) had been eliminated and transformed into liquids and gases (low-molecular-weight) instead of biochar (Zhao et al. 2017; Zhang et al. 2015; Ronsse et al. 2013). Additionally, Table 1 showed the percentages of moisture, ash, volatile gases, and fixed carbon contents for both raw feedstock (SS) and its biochar types (T1 and T2). The moisture content of SS was relatively low, less than 10%. This percentage is favorable for the pyrolysis process (Khairy et al. 2023). However, the moisture contents of both T1 and T2 were relatively close and lower than SS by about 50%. The opposite trend was observed in the case of ash contents (%), which were higher in both biochar types than their raw feedstock by about 30% (on average). The ash content of T1 was slightly higher than that of T2 by about 8.57%. This is consistent with previous studies, which confirms that the higher the pyrolysis temperature, the higher the ash content. Many researchers have confirmed the same finding using different biochar materials produced at different temperatures (Zhao et al. 2017; El-Gamal et al. 2017; Venkatesh et al. 2022). The fixed carbon content was increased about 2.5 and 2.8 times for T1 and T2 relative to SS, respectively. This increase is clearly observed with the decreased volatile gasses due to the depletion of volatile elements compared to the volatile gasses of the SS material.
The calculated C, H, and O mass fractions in sesame feedstock and biochar are presented in Table 1. There is a difference in the calculated value of C and O contents of SS relative to that obtained by the CHNS analyzer in our previous study (El-Gamal et al. 2022), while the H content was very close for both methods. Carbon content increased by about 5.19% and oxygen content decreased by about 8.56%. Regardless of that observation relative to SS, the carbon mass fraction was higher in both biochar about 11.74% and 15.91% for T1 and T2, respectively. Additionally, the percentages of H and O in both biochars were lower than those in the sesame feedstock. Generally, both biochars occupied close values for carbon, hydrogen, and oxygen. That means both pyrolysis temperature and residence time have a significant effect on the contents of C, H, and O that affect the biochar structure through various reactions during the pyrolysis process such as polymerization, condensation, dehydration, and aromatization (Venkatesh et al. 2022).
The mole ratios of H/C and O/C, an indicator of the degree of biochar stability in soil, were calculated using mathematical Eqs. (6) and (7). The estimation data in Table 1 indicated that the pyrolysis temperature declined the ratios of H/C and O/C in both biochar and sesame stalk feedstock due to the loss of degradable polar contents (Mierzwa-Hersztek et al. 2019; Venkatesh et al. 2022; El-Gamal et al. 2022). Both biochars had H/C and O/C ratios below 1.00 and 0.4, respectively, indicating the formation of aromatic compounds. The lower the H/C molar ratio, the higher the carbonization and structural stability of biochar. However, H is the essential element in raw organic components, so the H/C mole ratio is higher in SS. Several reactions occur during pyrolysis that eliminate hydrogen and decrease its value in biochar (e.g., cleavage and cracking of weak hydrogen bonds, dehydration, and dehydrogenation). Additionally, the reduction in the O/C mole ratio leads to a decrease in the decomposition rate and a rise in the aromatic rings in the biochar structure that occur through the elimination of oxygen through dehydration and decarboxylation reactions during the pyrolysis process (Venkatesh et al. 2022). The lower ratios of H/C and O/C improve the chemical stability of biochar and increase resistance against microbial degradation that persists long-term in soil for hundreds of years, making it appropriate for soil applications. However, the data proved that the pyrolysis conditions conducted in this study were successfully converted sesame stalk feedstock into biochar using the proposed solar reactor (Venkatesh et al. 2022).
Approximately, both biochar types (T1 and T2) had the same pH in the alkaline range (9.7–9.77), with a slight increase in T1. While the pH value of raw feedstock is in the acidic range (5.36). Longer pyrolysis processes with higher temperatures produce more alkaline biochar with a high ash content due to the formation of carbonates and the contents of inorganic alkalis (K, Na, Ca, and Mg). Furthermore, higher pyrolysis temperatures cause acidic functional groups to be depleted and basic functional groups formed (Ronsse et al. 2013; Zhao et al. 2017). It has been reported that during pyrolysis at temperatures above 300 °C, the alkali salts are distinct from the organic compounds (Tomczyk et al. 2020; Venkatesh et al. 2022). In our previous study, the pH values of sesame stalk biochar produced at 300 °C were near neutral (El-Gamal et al. 2022). Additionally, numerous researchers confirmed that high pyrolysis temperatures increased biochar ash content, pH value, and surface area (El-Gamal et al. 2017; Chatterjee et al. 2020; Tu et al. 2022).
The highest surface area (BET) (4.2437 ± 0.091 m2 g−1, n = 3) was observed in T2 biochar compared to T1 biochar (1.3345 ± 0.101 m2 g−1, n = 3). In T1, with the higher pyrolysis temperatures and shorter residence time concerning T2 pyrolysis conditions, the surface area of T1 decreased three times with respect to T2 biochar, while the total pore volume (TPV) increased by 89% in T2 compared to T1. The opposite trend was observed in the case of mean pore diameter (MPD) that increased by 68% in T1, probably due to the increased pyrolysis temperature that raised the emission of volatile gases because of the thermal degradation of lignocellulosic compounds. El-Gamal et al. (2017) reported that the specific surface area (SA, 154.7 m2 g−1) and pore size (PS, 0.1 m2 g−1) of rice husks-biochar were lower than sugarcane-biochar (SA, 185.6 m2 g−1 and PS, 0.1 m2 g−1). El-Gamal et al. (2022) reported that the negative charge of sesame stalk feedstock was − 14.3 mV, which was lower than the produced biochar. The higher negative charges of surface biochar types indicated by the zeta potential (ZP) results in T1 (− 20.7 mV) than in T2 (− 17.9 mV) are attributed to the increased temperatures during the pyrolysis processes. It means that the higher the pyrolysis temperature, the lower the electronegativity. Generally, increasing the pH leads to an increase in the negative surface charges of biochar. The particle size distribution by the intensity of sesame stalk feedstock (SS) and its two biochar types (T1 and T2) is shown in Fig. 4. The average diameter of SS was around 421.7 nm higher than T1 and T2, which were around 277.6 nm and 341.1 nm, respectively. Generally, the particle size distribution by the intensity of SS was 473.7 nm (75.4%), 4958 nm (13.2%), and 102.2 nm (11.3%). This diameter decreased in both biochar types to 254.9 nm (94.3%) and 5211 nm (5.7%) for T1 and 379.2 nm (100%) for T2. A positive relationship has been observed between the BET of the produced biochar and its particle size. This means increasing pyrolysis temperature led to a decreas the biochar particle size on average, which appeared to be highly stable with zeta potential values and surface area. The same observation was presented by Riva et al. (2019) who figured the particle size of biochar produced at 500, 800, and 1100 ℃. They found that the higher the pyrolysis temperature, the smaller the particle diameter.
FTIR
The surface functional groups of the sesame stalk biomass and yielded biochar types detected using FT-IR spectroscopy can be seen in Fig. 5. FT-IR figures were generated from Origin Pro 8 SRI v8.0773 (B773) software. The raw sesame stalk (SS) and two biochar types (T1 and T2) had the same peaks with a noticeable change in peak intensities, indicating the effect of pyrolysis time and temperature on the surface functional groups. The broad peak is around 3440 cm−1 of H–O stretching vibration relates to alcohol and hydroxyl compounds, indicating the presence of water and hydroxyl (Elnour et al. 2019; Tsai et al. 2021; El-Gamal et al. 2022). The bands of C–H stretching vibrations of the methylene group (aliphatic compounds) were observed at 2915–2944 cm−1. These peaks correspond to cellulose and hemicellulose compounds in their raw material that became weak in biochar due to thermal degradation and dehydration of the aliphatic structures at a temperature above 350 ℃ during the pyrolysis process (Zhao et al. 2020). The band at 1730 cm−1 is related to carbonyl compounds, carboxylic acids, ketones, and ester groups of hemicellulosic compounds. This band vanished in the two biochars, which is attributable to the effect of pyrolysis temperature and thermal on the breakdown of these compounds (El-Gamal et al. 2022). The peak at 1602 cm−1 presented in SS was shifted in T1 and T2to be at 1594–1588 cm−1 and became stronger, which are attributed to aromatic rings (–C=C–) indicating the presence of benzene-like rings due to the degradation and rearrangement of carbon atoms in lignocellulosic structure (Chatterjee et al. 2020). The aromatic C=C stretching peaks appeared around 1429 cm−1, suggesting an increase in the aromaticity at high temperatures and a decrease in aliphatic hydrocarbons (El-Gamal et al. 2017; Tsai et al. 2021). The 1030–1113 cm−1 peaks are associated with the C–O bond’s stretching vibration and may be attributed to the alcoholic and carboxylic groups found in lignocellulose compounds (Zhao et al. 2020 and Stylianou et al. 2020). It is observed that the peak at 1400 cm−1 is of carboxyl-carbonate structures and CH (El-Gamal et al. 2017). This is because of the thermal degradation of carbonyl and aliphatic groups during the pyrolysis process (Yang et al. 2020). In addition, Yang et al. (2020) discussed that the nonpolar functional groups of the alkyl C–H and C=C bonds are broken and converted to more hydrophilic properties at higher temperatures ranging from 400 to 500 ℃. While carboxylic and carbonyl groups (O-containing groups) decrease at 350–500 ℃ temperature range. The bands at 610 and 617 cm−1 indicate the presence of alkyl halides or inorganic compounds (Claoston et al. 2014; El-Gamal et al. 2017). The peak related to C–H bonds at 873 cm−1 was observed in the generated biochar types (T1 and T2) rather than that produced using a parabolic dish reactor (300 ℃) of the same material in our previous study (El-Gamal et al. 2022). Biochar produced at temperatures below 350 ℃ contains a wide range of functional groups, including O-containing groups (hydroxyl, carboxyl, and carbonyl) and a significant amount of alkyl –CH3, C–H, and C=C bonds. These groups typically decrease with increasing pyrolysis temperatures due to the destruction of cellulosic compounds (Yang et al. 2020).
Scanning electron microscopy (SEM)
The SEM images of the sesame stalk feedstock (SS) and its biochar samples (T1 and T2) are shown in Fig. 6. The SS sample had a smaller pore size, a relatively lower porosity, and a blocked tubular structure compared to its biochar. These morphological structures were completely altered due to the removal of easily degradable organic substances in the form of volatile gases during the pyrolysis process. Compared to sesame stalk biomass, a highly complex network with sharp-edged particles of pores at X500 can be observed for both biochar samples. The high magnification images at X2000 clearly display the porous structures with well-developed pores in the biochar samples. The appearance of deep channels and pores became more prominent with increasing pyrolysis temperatures caused by organic materials volatilization. However, it is observed that the presence of small particles on the T1 surface and fills the cavities of porous structures, indicating the presence of ash on its surface. The same observation was supported by Fernandes et al. (2020) who noted that rising pyrolysis temperatures caused a buildup of ash particles on the biochar surface and clogged its micropores, resulting in a reduction in biochar-specific surface area and total pore volume, which conformed with surface area and total pore volumes in T1 (Table 1). Comparing with our previous results in El-Gamal et al. (2022), we observed that there is a gradual change in the surface morphology and pore structures of the created biochar due to the pyrolysis temperature increase from 300 °C (the previous study) to 470 and 420 °C (current study) for T1 and T2, respectively. Moreover, the higher temperatures of biochar production develop surface morphology, changes in the pore structure, and increased pore numbers. However, increasing pyrolysis temperature leads to rearranging the molecular structure of the generated biochar due to the rise in the degradation of cellulosic, hemicellulosic, and lignin compounds and the dehydrogenation of methylene groups, which produce more stable aromatic compounds, and consequently, more recalcitrant biochar is produced (Jindo et al. 2014; El-Gamal et al. 2017; Sedláková et al. 2021). The decomposition of cellulose and hemicellulose during pyrolysis occurs between 250 ℃ and 400 ℃, as well as lignin, which decomposes at temperatures higher than 300–550 ℃ (Kan et al. 2016). These confirmed that the proposed solar disk reactor was better than that suggested in our previous study.
Because of its cheapness and the variety of raw materials used to produce biochar, biochar is more suitable for carbon fixation, soil conditioners, and sorbent materials to remove organic and inorganic pollutants. It is considered a valuable biomaterial containing a wide range of plant macro- and micro-nutrients that could be used as soil amendments. The concentration of these nutrients depends on the biomass type and pyrolysis conditions. Raising pyrolysis temperature increases mass loss, subsequently increasing ash content (nutrient elements) in the biochar (Kan et al. 2016). In addition, it has unique characteristics such as a sizable surface area, huge channels and pores in different nano-size diameters, a variety of surface functional groups, and a high cation exchange capacity (Tomczyk et al. 2020), which could be suitable for removing different toxic organic and inorganic materials from wastewater. Yin et al. (2018) concluded that sesame straw biochar has enormous potential to adsorb NH4+ and PO43− from eutrophic water. They reported that the pyrolysis temperature had influenced the adsorption capacity of sesame biochar. The pyrolysis temperature up to 700 ℃ was the best to remove PO43− due to the high surface area and high content of Ca, Mg, and Al. At the same time, sesame biochar pyrolyzed at a lower temperature (300 ℃) preferred to adsorb NH4+ because of the abundant functional groups. Furthermore, the nutrient-loaded biochar (N or P) could be applied to agricultural soil as a slow-release fertilizer. Another study conducted by El-Gamal et al. (2023) to eliminate multi-metal ions (Zn2+, Cu2+, Mn2+, and Fe2+) as micronutrients from water by rice husk biochar (RHB) pyrolyzed at temperatures ranging from 350 to 650. They found that RHB (450) could be effectively used to retain multi-metal-ions and their removal efficiency reached 86.38% (Zn2+), 87.61% (Cu2+), 76.26% (Mn2+), and 65.90% (Fe2+). Additionally, compared to the same treatment of rice husk feedstock, the bioavailability of these micronutrients in incubated calcareous sandy soil amended with RHB (450) loaded metals was superior for long-term application. That reaffirmed the advantages of adding biochar-loaded nutrients to the soil as a slow-release fertilizer.
Conclusion
This study aims to investigate the physicochemical properties of sesame biochar obtained from different conditions of pyrolysis processes (470 °C for one hour and 440 °C for two hours) using a modified disk reactor mounted on the solar parabolic dish collector. During the pyrolysis operation, the solar radiation brightness degree varied, which significantly affected the reactor's surface temperature. Biochar formation during the pyrolysis process is directly influenced by pyrolysis temperature and duration. The results obtained for both sesame stalk biochar types remarkably changed after pyrolysis of feedstock, indicating a reduction in the amount of volatile matter, hydrogen, oxygen contents, and molar ratios of H/C (< 1) and O/C (< 0.4). On the other hand, percentages of carbon, ash, fixed carbon contents, and the surface negative charge increased relative to their raw feedstock. The lower ratios of H/C and O/C and a higher percentage of fixed carbon improve the chemical stability of biochar while increasing resistance against microbial degradation that persists long-term in soil for hundreds of years, making it appropriate for soil applications. In general, the physicochemical properties of the produced biochar were relatively close regardless of BET, which was significantly influenced by pyrolysis time rather than temperature. However, the temperature of the suggested reactor showed an even distribution. The data demonstrated that the pyrolysis conditions used in this study effectively converted sesame stalk feedstock into biochar using the suggested solar reactor. Finally, the biochar obtained by the proposed solar parabolic dish collector is reliable and suitable to be used as sorbent material to eliminate pollutants from wastewater and contaminated soils, as well as soil amendments and ameliorants.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available at the time being, because they are in preparation and analyses for further publication(s) within the framework of the research project 33,541. However, the corresponding author may provide access to some of the data upon reasonable request.
References
Cárdenas-Aguiar E, Gascó G, Paz-Ferreiro J, Méndez A (2017) The effect of biochar and compost from urban organic waste on plant biomass and properties of an artificially copper polluted soil. Int Biodeterior Biodegrad 124:223–232. https://doi.org/10.1016/j.ibiod.2017.05.014
Chatterjee R, Sajjadi B, Chen W-Y, Mattern DL, Hammer N, Raman V, Dorris A (2020) Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front Energy Res 8:85. https://doi.org/10.3389/fenrg.2020.00085
Chintala V, Kumar S, Pandey JK, Sharma AK, Kumar S (2017) Solar thermal pyrolysis of non-edible seeds to biofuels and their feasibility assessment. Energy Convers Manag 153:482–492. https://doi.org/10.1016/j.enconman.2017.10.029
Claoston N, Samsuri A, Husni MA, Amran MM (2014) Effects of pyrolysis temperature on thephysicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag Res 32:331–339. https://doi.org/10.1177/0734242X14525822
Ebaid HM, Farag HA, El Falaky AA (2016) Using GIS and remote sensing approaches to delineate potential areas for runoff management applications in Egypt. Int J Environ Sci Eng 7:85–93
Elbadrashini NKA, El-Eraky MB, Abd Elmoneim SM, El-sawy MA (2019) Possibility of using agricultural waste in Egypt “a case study in Gharbia governorate.” Arab Univ J Agric Sci Ain Shams Univ Cairo Egypt 27:1739–1759
El-Gamal EH, Emran M, Elsamni O, Rashad M, Mokhiamar O (2022) Parabolic dish collector as a new approach for biochar production: an evaluation study. Appl Sci 12:12677. https://doi.org/10.3390/app122412677
El-Gamal EH, Saleh M, Elsokkary I, Rashad M, Abd El-Latif MM (2017) Comparison between properties of biochar produced by traditional and controlled pyrolysis. Alex Sci Exch J 38:412–425. https://doi.org/10.21608/asejaiqjsae.2017.3720
El-Gamal EH, Salem LR, Mahmoud AH, Saleh ME (2023) Evaluation of rice husk biochar as a micronutrients carrier on micronutrients availability in a calcareous sandy soil. J Soil Sci Plant Nutr 23:1633–1647. https://doi.org/10.1007/s42729-023-01184-9
Elnour AY, Alghyamah AA, Shaikh HM, Poulose AM, Al-Zahrani SM, Anis A, Al-Wabel MI (2019) Effect of pyrolysis temperature on biochar microstructural evolution, physicochemical characteristics, and its influence on biochar/polypropylene composites. Appl Sci 9:1149. https://doi.org/10.3390/app9061149
Fangary A (2021) An economic study of the sesame crop in Egypt. Sci J Agric Sci 3:245–258. https://doi.org/10.21608/sjas.2021.69073.1080
Fernandes CBC, Mendes FK, Júnior DAZ, Caldeira D-SVP, Teófilo D-STM, Silva ST, Mendonça V, Souza D-FM, Silva VD (2020) Impact of pyrolysis temperature on the properties of eucalyptus wood-derived biochar. Mater 13:5841. https://doi.org/10.3390/ma13245841
Hong M, Zhang L, Tan Z, Huang Q (2019) Effect mechanism of biochar’s zeta potential on farmland soil’s cadmium immobilization. Environ Sci Pollut Res 26:19738–19748. https://doi.org/10.1007/s11356-019-05298-5
Jindo K, Mizumoto H, Sawada Y, Sanchez-Monedero MA, Sonoki T (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 11:6613–6621. https://doi.org/10.5194/bg-11-6613-2014
Jouhara H, Ahmad D, Van Den Boogaert I, Katsou E, Simons S, Spencer N (2018) Pyrolysis of domestic based feedstock at temperatures up to 300 C. Therm Sci Eng Prog 5:117–143. https://doi.org/10.1016/j.tsep.2017.11.007
Kan T, Strezov V, Evans TJ (2016) Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew Sustain Energy Rev 57:1126–1140
Khairy M, Amer M, Ibrahim M, Ookawara S, Sekiguchi H, Elwardany A (2023) The influence of torrefaction on the biochar characteristics produced from sesame stalks and bean husk. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-03822-9
Khalil AK, Mubarak AM, Kaseb SA (2010) Road map for renewable energy research and development in Egypt. J Adv Res 1:29–38. https://doi.org/10.1016/j.jare.2010.02.003
Klasson KT (2017) Biochar characterization and a method for estimating biochar quality from proximate analysis results. Biomass Bioenerg 96:50–58. https://doi.org/10.1016/j.biombioe.2016.10.011
Laghari M, Mirjat MS, Hu Z, Fazal S, Xiao B, Hu M, Chen Z, Guo D (2015) Effects of biochar application rate on sandy desert soil properties and sorghum growth. CATENA 135:313–320
Lehmann J, Rilig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar affects on soil biota—a review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Lewandowski WM, Januszewicz K, Kosakowski W (2019) Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—a review. J Anal Appl Pyrolysis 140:25–53. https://doi.org/10.1016/j.jaap.2019.03.018
Masulili A, Utomo WH, Syechfani MS (2010) Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia. J Agric Sci 2:39–47
Mierzwa-Hersztek M, Gondek K, Jewiarz M, Dziedzic K (2019) Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J Mater Cycles Waste Manag 21:786–800. https://doi.org/10.1007/s10163-019-00832-6
Ndukwu MC, Horsfall IT, Ubouh EA, Orji FN, Ekop IE, Ezejiofor NR (2021) Review of solar-biomass pyrolysis systems: focus on the configuration of thermal-solar systems and reactor orientation. J King Saud Univ Eng Sci 33:413–423. https://doi.org/10.1016/j.jksues.2020.05.004
Park JH, Ok YS, Kim SH, Cho JS, Heo JS, Delaune RD, Seo DC (2015) Evaluation of phosphorus adsorption capacity of sesame straw biochar on aqueous solution: influence of activation methods and pyrolysis temperatures. Environ Geochem Health 37:969–983. https://doi.org/10.1007/s10653-015-9709-9
Riva L, Surup GR, Buø TV, Nielsen HK (2019) A study of densified biochar as carbon source in the silicon and ferrosilicon production. Energy 181:985–996. https://doi.org/10.1016/j.energy.2019.06.013
Ronsse F, Van Hecke S, Dickinson D, Prins W (2013) Production and characterization of slow pyrolysis biochar: influence of feedstock type and pyrolysis conditions. GCB Bioenergy 5:104–115. https://doi.org/10.1111/gcbb.12018
Rony AH, Mosiman D, Sun Z, Qin D, Zheng Y, Boman JH, Fan M (2018) A novel solar powered biomass pyrolysis reactor for producing fuels and chemicals. J Anal Appl Pyrolysis 132:19–32. https://doi.org/10.1016/j.jaap.2018.03.020
Said N, El-Shatoury SA, Díaz LF, Zamorano M (2013) Quantitative appraisal of biomass resources and their energy potential in Egypt. Renew Sust Energ Rev 24:84–91. https://doi.org/10.1016/j.rser.2013.03.014
Samsuri AW, Sadegh-Zadeh F, Seh-Bardan BJ (2014) Characterization of biochars produced from oil palm and rice husks and their adsorption capacities for heavy metals. Int J Environ Sci Technol 11:967–976
Sedláková M, Száková J, Lhotka M, Hailegnaw NS, Holečková Z, Pračke K, Robledo-Mahón T, Tlustoš P (2021) Changes in soil carbon and nitrogen accessibility with the application of biochars with different morphological and physical characteristics. J Soils Sediment 21:1644–1658. https://doi.org/10.1007/S11368-021-02910-5
Shaw I (2003) The Oxford history of ancient Egypt. OUP Oxford, Oxford
Stylianou M, Christou A, Dalias P, Polycarpou P, Michael C, Agapiou A, Papanastasiou P, Fatta-Kassinos D (2020) Physicochemical and structural characterization of biochar derived from the pyrolysis of biosolids, cattle manure and spent coffee grounds. J Energy Inst 93:2063–2073
Sun J, He F, Pan Y, Zhang Z (2017) Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types. Acta Agr Scand 67:12–22. https://doi.org/10.1080/09064710.2016.1214745
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Tsai W-T, Lin Y-Q, Huang H-J (2021) Valorization of rice husk for the production of porous biochar materials. Ferment Artic 7:1–12. https://doi.org/10.3390/fermentation7020070
Tu P, Zhang G, Wei G, Li J, Li Y, Deng L, Yuan H (2022) Influence of pyrolysis temperature on the physicochemical properties of biochars obtained from herbaceous and woody plants. Bioresour Bioprocess. https://doi.org/10.1186/s40643-022-00618-z
Venkatesh G, Gopinath KA, Reddy KS, Reddy BS, Prabhakar M, Srinivasarao C, Visha Kumari V, Singh VK (2022) Characterization of biochar derived from crop residues for soil amendment, carbon sequestration and energy use. Sustain 14:2295. https://doi.org/10.3390/su14042295
Wan J, Lin L, Ayub SK, Zhang W, Shen G, Hu S, Qian X (2020) Characterization and adsorption performance of biochars derived from three, key biomass constituents. Fuel 269:117142. https://doi.org/10.1016/j.fuel.2020.117142
Wang T, Camps-Arbestain M, Hedley M, Singh BP, Calvelo-Pereira R, Wang CY (2014) Determination of carbonate-C in biochars. Soil Res 52:495–504. https://doi.org/10.1071/SR13177
Xie T, Reddy KR, Wang C, Yargicoglu E, Spokas K (2015) Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Environ Sci Technol 45:939–969. https://doi.org/10.1080/10643389.2014.924180
Yang W, Shang J, Li B, Flury M (2020) Surface and colloid properties of biochar and implications for transport in porous media. Crit Rev Environ Sci Technol 50:2484–2522. https://doi.org/10.1080/10643389.2019.1699381
Yin Q, Zhang B, Wang R, Zhao Z (2018) Phosphate and ammonium adsorption of sesame straw biochars produced at different pyrolysis temperatures. Environ Sci Pollut Res 25:4320–4329. https://doi.org/10.1007/s11356-017-0778-4
Zaman CZ, Pal K, Yehye WA, SagadevanS SST, Adebisi GA, Marliana E, Rafique RF, Johan RB (2017) Pyrolysis: a sustainable way to generate energy from waste. INTECH. https://doi.org/10.5772/intechopen.69036
Zhang J, Liu J, Liu R (2015) Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour Technol 176:288–291. https://doi.org/10.1016/j.biortech.2014.11.011
Zhao M, Dai Y, Zhang M, Feng C, Qin B, Zhang W, Zhao N, Li Y, Ni Z, Xu Z, Tsang DCW, Qiu R (2020) Mechanisms of Pb and/or Zn adsorption by different biochars: biochar characteristics, stability, and binding energies. Sci Total Environ 717:136894. https://doi.org/10.1016/j.scitotenv.2020.136894
Zhao S-X, Ta N, Wang X-D (2017) Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 10:1293. https://doi.org/10.3390/en10091293
Acknowledgements
The authors acknowledge the support provided by Science, Technology & Innovation Funding Authority (STIFA), project ID. 33541.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided byThe Science, Technology& Innovation Funding Authority (STDF) in cooperation with The Egyption Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization and design were performed by MR, OE; OM; ME, and EHE-G. Methodology was given by OE, MR, and OM. Material preparation, data collection and formal analysis were performed by OM, ME, and EHE-G. Software was done by EHE-G. Visualization was presented by OE, MR, and OM. Supervision was done by OE, MR, and OM. The original draft of the manuscript was written by EHE-G and ME, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emran, M., El-Gamal, E.H., Mokhiamar, O. et al. A novel solar disk chamber reactor for agricultural waste recycling and biochar production. Clean Techn Environ Policy 26, 467–479 (2024). https://doi.org/10.1007/s10098-023-02635-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-023-02635-8