Abstract
In recent years, there has been a great interest in the conversion of lignocellulosic structures to furfural. There are many technologies available for this process. Nonetheless, the present work reports for the first time the use of pectin, a non-lignocellulosic structure, for furfural production. The pectin was extracted from food industry waste derived from cactuses, orange peels and mangoes peels. The extracted pectins were analyzed by infrared spectroscopy (ATR–FTIR) in order to evaluate the degree of esterification (DE). The high DE influences in the hydrolysis reaction in the following stages: (1) hydration, it allows a fast glycosidic bond cleavage in the polysaccharide. (2) Dehydration, an intermediary step in the furfural production from galacturonic acid. The Maillard reaction herein reported not only is used in a novel way to produce furfural but also it has been modified to be performed in acidic conditions to increase the furfural production rate. From the evaluated reactions, it was found that the highest furfural production was obtained with manila mango pectin (82.6 g/L) with a DE of 51.2%. These findings demonstrate that pectins with DE below 75%, the minimum value to be considered for applications in the food and pharmaceutical industries, could be applied for the generation of furfural, a chemical platform for the production of chemicals and biofuels.








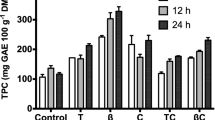
modified Maillard reaction in H2SO4 for cactus pectin (120 min)
Similar content being viewed by others
Abbreviations
- DE:
-
Degree of esterification
- GalA.:
-
Galacturonic acid
- K.P.:
-
Kent mango peels pectin
- M.P.:
-
Manila mango peels pectin
- C.P.:
-
Cactus pectin
- O.P.:
-
Orange peels pectin
- A.P.:
-
Apple pectin
- RH.:
-
Relative humidity
References
Alba K, Laws AP, Kontogiorgos V (2015) Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll 43:726–735
Asadova PG, Guseinov GZ, Allakhverdiev MA (2013) The reaction of condensation of furfural with mercaptoacetic acid esters. Rus J Appl Chem 86(5):787–789
Badui DS (2006) Químicas de los alimentos. Cuarta Edición. ISBN: 970-26-0670-5
Baxter JH (1995) Free amino acid stability in reducing sugar systems. J Food Sci 60(2):405–408
Bichara LC, Alvarez PE, Fiori Bimbi MV et al (2016) Structural and spectroscopy study of a pectin isolated from citrus peel by using FTIR and FT-Raman spectra and DFT calculations. Infrared Phys Technol. https://doi.org/10.1016/j.infrared.2016.03.009
Bicu I, Mustata F (2013) Optimization of isolation of cellulose from orange peel using sodium hydroxide and chelating agents. Carbohydr Polym 98:341–348
Burana-osot J, Soonthornchareonnon N, Hosoyama S et al (2010) Partial depolymerization of pectin by a photochemical reaction. Carbohydr Res 345:1205–1210
Cai L, Li D, Dong Z et al (2015) Change regularity of the characteristics of Maillard reaction products derived from xylose and Chinese shrimp waste hydrolysates. LWT Food Sci Technol. https://doi.org/10.1016/j.lwt.2015.09.007
Carvalho DO, Correia E, Lopes L et al (2004) Further insights into the role of melanoidins on the antioxidant potential of barley malt. Food Chem 160:127–133
Ciriminna R, Chavarría-Hernández N, Rodríguez Hernández AI (2015) Pectin: a new perspective from the biorefinery standpoint. Biofuels Bioprod Biorefining 9:368–377
Dumitriu S (2004) Polysaccharides: structural diversity and functional versatility, 2nd edn. CRC Press, ISBN 9781420030822
Edwards M, Doran-Peterson J (2012) Pectin-rich biomass as feedstock for fuel ethanol production. Appl Microbiol Biotechnol 95:565–575
Espino-Díaz M, Ornelas-Paz JJ, Martínez-Téllez MA et al (2010) Development and characterization of edible films based on mucilage of Opuntia ficus-indica (L.). J Food Sci 75(6):E347–E352. https://doi.org/10.1111/j.1750-3841.2010.01661.x
Gnanasambandam R, Proctor A (2000) Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem 68:327–332
Helou C, Jacolot P, Niquet-Léridon C et al (2016) Maillard reaction products in bread: a novel semi-quantitative method for evaluating melanoidins in bread. Food Chem 190:904–911
Hongsiri W, Danon B, Jong W (2015) The effects of combined catalysis of oxalic and seawater on the kinetics of xylose and arabinose dehydration to furfural. Int J Energy Environ Eng 6:21–30
Hua D, Wu Y, Liu Y et al (2016) Preparation of furfural and reaction kinetics of xylose dehydration to furfural in high-temperature water. Pet Sci. https://doi.org/10.1007/s12182-015-0069-y
Karnik D, Jung J, Hawking S et al (2016) Sugar beet pectin fractionated using isopropanol differs in galacturonic acid, protein, ferulic acid and surface hydrophobicity. Food Hydrocoll 60:179–185
Kyomugasho C, Christiaens S, Shpigelman A et al (2013) FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit-and vegetable-based matrices. Food Chem. https://doi.org/10.1016/j.foodchem.2014.12.033
Ledl F, Schleicher E (1990) New aspects of the Maillard reaction in foods and in the human body. Angew Chem Int Ed Engl 29(6):565–594
Li H, Chen X, Ren J et al (2015) Functional relationship of furfural yields and the hemicellulose-derived sugars in the hydrolysates from corncob by microwave-assisted hydrothermal pretreatment. Biotechnol Biofuels. https://doi.org/10.1186/s13068-015-0314-z
Loow YL, Wu TY, Tan KA, Lim YS et al (2015) Recent advances in the application of inorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J Agric Food Chem 63(38):8349–8363
Loow YL, Wu TY, Jahim JM, Mohammad AW, Teoh WH (2016) Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 23(3):1491–1520
Maran JP, Sivakumar V, Thirugnanasambandham K et al (2013) Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr Polym 97:703–709
Mestdagh F, De Wilde T, Castelein P et al (2008) Impact of the reducing sugars on the relationship between acrylamide and Maillard browning in French fries. Eur Food Res Technol 227:69–76
Müller-Maatsch J, Bencivenni M, Caligiani A et al (2015) Pectin content and composition from different food waste streams. Food Chem. https://doi.org/10.1016/j.foodchem.2016.01.012
Nur Meinita MD, Hong YK, Jeong GT (2012) Comparison of sulfuric and hydrochloric as catalysts in hydrolysis of Kappaphycus alvarezii (cottonni). Bioprocess Biosyst Eng 35:123–128
Oliveira TIS, Rosa MF, Cavalcante FL et al (2015) Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. https://doi.org/10.1016/j.foodchem.2015.08.080
Pereira PHF, Oliveira TIS, Rosa MF et al (2016) Pectin extraction from pomegranate peels with citric acid. Int J Biol Macromol 88:373–379. https://doi.org/10.1016/j.ijbiomac.2016.03.074
Sayah MY, Chabir R, Benyahia H et al (2016) Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS ONE 11:e0161751. https://doi.org/10.1371/journal.pone.0161751
Shi J, Mazza G, Maguer M (2002) Functional foods: biochemical and processing aspects. Fla, Boca Raton
Singthong J, Ningsanond S, Cui S, Goff H (2005) Extraction and physicochemical characterization of Krueo Ma Noy pectin. Food Hydrocoll 19:793–801
Srivastava P, Malviya R (2011) Extraction, characterization and evaluation of orange peel waste derived pectin as a pharmaceutical excipient. Nat Prod J 1:65–70
Stankovikj F, Garcia-Perez M (2017) TG-FTIR method for the characterization of bio-oils in chemical families. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.6b03132
Tarabanko VE, Chernyak MY, Simakova IL et al (2015) Antiknock properties of furfural derivatives. Organic synthesis and industrial organic chemistry. Rus J Appl Chem 88(11):1778–1782. https://doi.org/10.1134/s10704272150110063
Tas NG, Gökmen V (2015) Effect of alkalization on the Maillard reaction products formed in cocoa during roasting. Food Res Int. https://doi.org/10.1016/j.foodres.2015.12.021
Urias-Orona V, Rascón-Chu A, Lizardi-Mendoza J et al (2010) A novel pectin material: extraction, characterization and gelling properties. Int J Mol Sci 11:3686–3695
Vanderhaegen B, Neven H, Verstrepen KJ et al (2004) Influence of the brewing process on furfuryl ethyl ether formation during beer aging. J Agric Food Chem 52:6755–6764
Verzelloni E, Tagliazucchi D, Conte A (2010) From balsamic to healthy: traditional balsamic vinegar melanoidins inhibit lipid peroxidation during simulated gastric digestion of meat. Food Chem Toxicol 48:2097–2102
Wang H, Qian H, Yao W (2011) Melanoidins produced by the Maillard reaction: structure and biological activity. Food Chem 128:573–584
Wang S, Lin H, Zhang L et al (2016a) Structural characterization and pyrolysis behavior of cellulose and hemicellulose isolated from softwood pinus armandii franch. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.6b00650
Wang W, Ma X, Jiang P, Hu L et al (2016b) Characterization of pectin from grapefruit peel: a comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2016.06.019
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Zeitsch KJ (2000) The chemistry and technology of furfural and its many by-products. Köln, Germany
Zouambia Y, Ettoumi KY, Krea M et al (2014) A new approach for pectin extraction: electromagnetic induction heating. Arab J Chem. https://doi.org/10.1016/j.arabjc.2014.11.011
Acknowledgements
The authors are grateful to Ing. Rubén Palomino Luna for his contribution with biomasses for this study. The first author wishes to thank colleague and friend Jessica Marquez León Ph.D. for supporting the write-up with inspiration and motivation, and thanks to Consejo Nacional de Ciencia y Tecnología (CONACyT, Ph.D scholarship #233787). The authors would also like to thank Katlin B Stivers for her contributions to language editing and proofreading.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
López-Mercado, J., Nambo, A., Toribio-Nava, ME. et al. High and low esterification degree pectins decomposition by hydrolysis and modified Maillard reactions for furfural production. Clean Techn Environ Policy 20, 1413–1422 (2018). https://doi.org/10.1007/s10098-018-1570-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-018-1570-y