Abstract
Factors influencing dehydration of xylose to furfural, such as catalyst and extract agents, were investigated. Results indicated that high-temperature water may substitute for solid and liquid acid as a catalyst, and ethyl butyrate improved furfural yield for the high distribution coefficient. A furfural yield of 75 % was obtained at 200 °C for 3 h in ethyl butyrate/water. The reaction kinetics of xylose dehydration to furfural was investigated and it was found that the reaction order was 0.5, and the activation energy was 68.5 kJ/mol. The rate constant k showed a clear agreement with the Arrhenius law from 160 to 200 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
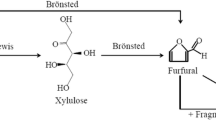
Furfural, which is readily obtained from renewable biomass, is a key biomass-derived chemical that can be used to replace petrochemicals (Dias et al. 2005a; Lichtenthaler 1998). In addition, furfural is a common industrial solvent and an intermediate for preparation of fine-chemical products, resins/plastics, and biofuel (Climent et al. 2011; Li et al. 2014).
Furfural is conventionally produced by hydrolysis of a variety of biomass sources including almond shell, sugarcane, and corn with the aid of liquid acid catalysts at high temperatures (200–250 °C) (Kim et al. 2011). However, liquid acid catalysts are toxic and corrosive, and their applications can generate large amounts of toxic wastes. In point of the principles of green chemistry, the replacement of liquid acid catalysts with solid acid catalysts, such as zeolites (O’Neill et al. 2009) and acid salts (Dias et al. 2006), can overcome these drawbacks (Agirrezabal-Telleria et al. 2012a, b; Antunes et al. 2012). Additionally, solvent, which can rapidly and continuously remove furfural from an aqueous phase, is an important factor in increasing furfural yield. Zhang et al. (2012) reported that 1-butanol from biomass-based carbohydrates can be used as a renewable extraction solvent in a biphasic system on a MCM-41 catalyst, and 1-butanol can obviously increase the yield of furfural. A biphasic reactor system can easily separate furfural from an aqueous phase (Chheda et al. 2007). So researchers are paying more attention to green process for furfural preparation, and more rational catalytic systems have been developed recently. High-temperature water (HTW) was applied in the catalytic conversion of renewable lignocellulosic biomass to furfural (Akiya and Savage 2002). HTW can partly replace acid catalyst to produce high ionic products (Jing and LÜ 2007). Recently, kinetic studies of furfural formation have been conducted by using xylose or hemicellulose as a starting material on heterogeneous catalysts (Dias et al. 2005b; O’Neill et al. 2009), such as mineral acids (Marcotullio and De Jong 2010; Morinelly et al. 2009). Additionally, some methods to slow or inhibit side reactions (in Scheme 1) are studied by addition of extraction agent during reaction. As far as we know, biphasic systems are now applied, such as toluene/water (Shi et al. 2011a), dioxane/water (Chheda and Dumesic 2007), methyl isobutyl ketone/water (Moreau et al. 1996), and SBP/NaCl-DMSO (Li et al. 2015), but these organic solvents are toxic and from petrochemical sources; therefore, it is important to search for environment-friendly renewable solvents for preparation of furfural.
In this study, ethyl butyrate was selected and used as extraction agent to extract furfural from the aqueous phase. Then the effect of reaction conditions, such as temperature and catalyst, on xylose conversion and furfural yield was studied. The objective of the work was to find a green process for furfural preparation. In addition, the kinetic model of xylose dehydration to furfural was investigated in ethyl butyrate solvent.
2 Experimental
2.1 Preparation of catalyst
Organosulfonic acid-functionalized mesoporous silica (SO3H-SBA-15) was synthesized (Erdem et al. 2013; Hua et al. 2013; Shi et al. 2011b), and Tetraethoxysilane (TEOS) (Aldrich Co.) and copolymer Pluronic P123 (Aldrich Co.) were used as silica source and structure directing agent, respectively. Firstly, P123 (1.0 g, MW = 5800) was dissolved in 100 mL ethanol solution, and 1 mL TEOS and 0.4 g HCl (38 wt.%) were added to the solution, then 0.15 mL 3-mercaptopropyltrimethoxysilane (MPTMS) and 1 mL H2O2 (30 wt.%) were added and stirred at room temperature forwere added and stirred at room temperature for 20 h. After that, the mixture was evaporated using a rotary evaporator at 40 °C for 10 h, and then dried at 60 °C for 12 h, washed with ethanol, and dried at 60 °C again. At last, the template was removed from the as-synthesized material by washing with ethanol under reflux for 24 h. The sample was denoted as SBA-15–SO3H.
2.2 Analysis
All the extraction agents were from J&K Scientific Ltd. (China) and used without further purification. A 50-mL self-made autoclave with a stirrer was used. In a typical procedure, d-xylose (0.75 g, Sigma–Aldrich Co., 99 %), extraction agent (17.5 mL), and H2O (7.5 mL) were poured into the reactor (autoclave), and the reactor was heated to temperature and kept at the temperature for the desired time. Then the autoclave was cooled to room temperature. Products existed in aqueous and organic phases. The aqueous phase was examined using a high-performance liquid chromatograph (Shimadzu LC-20AD, Aminex HPX-87H column 300 × 7.8 (i.d.) mm, Japan) with 0.5 M H2SO4 as the mobile phase. The organic phase was determined using a gas chromatograph (Aigent 6820 and PEG-20 M, 30 m × 0.32 mm × 0.25 μm, USA).
The distribution coefficient of furfural between the organic and aqueous phases was determined as follows: 50 mL furfural solution (2 wt.%) and 50 mL extraction agent were fed into a 150-mL separatory funnel, and the mixture was vigorously shaken at room temperature, then stood for 7 h. The mixture was partitioned between an organic and aqueous phase, and the furfural masses in organic and aqueous phases were determined. The distribution coefficient (K) can be calculated by Eq. (1).
where W o and W A stand for furfural mass in organic and in aqueous phases, respectively.
The conversion of d-xylose (X), the selectivity of furfural (S), and the yield of furfural (Y) are calculated as follows.
where \(C_{\text{xylose}}^{s}\),\(C_{\text{xylose}}^{e}\), M furfural, and M stand for initial concentration of xylose, final concentration of xylose, mass of furfural, and mass of all the products, respectively.
3 Results and discussion
Table 1 shows the performance of HTW in preparation of furfural from xylose. The furfural yield of 50 % in HTW was attributed to the high value of K w (ion product of H2O) of HTW (Akiya and Savage 2002). The reason for the yield of no more than 50 % in HTW was attributed to the condensation reaction between xylose in the long residence time in HTW. The furfural yield was above 70 % in the biphasic system HTW/toluene, because furfural was immediately removed by toluene from the aqueous phase. Furfural selectivity was greatly improved as some side reactions were minimized at a high-volume ratio of toluene/water. HTW as a catalyst overcomes the shortcoming of liquid and solid acid catalysts.
In this work, ethyl butyrate, butyl acetate, n-butanol, n-butyl ether, and toluene solvents were used as extraction agents. Figure 1a shows the effect of extraction agent on furfural yield. The furfural yield was the lowest in n-butyl ether/HTW system, whereas the furfural yield was above 80 % in other four extraction agents. The furfural yield was the highest in ethyl butyrate/HTW system, which was related to the distribution coefficients of furfural in extraction agents (in Fig. 1b). The distribution coefficient (K) in ethyl butyrate was the highest, so the solubility of furfural was the highest in ethyl butyrate. Ethyl butyrate can remove furfural from an aqueous phase; hence, the furfural concentration in the aqueous phase was the lowest.
HTW has a potential as a catalyst because of its high ionic product (Promdej and Matsumura 2011), which is affected by temperature. Figure 2a shows the effect of temperature on furfural yield, furfural selectivity, and xylose conversion. The results show that the xylose conversion increased with temperature from 160 to 200 °C. Xylose conversion and furfural yield were 23 and 14 wt% at 160 °C, 95 and 75 wt% at 200 °C, respectively. This is attributed to the high ionic product of HTW at high temperature (Akiya and Savage 2002). At temperatures above 200 °C, the xylose conversion changed slightly, but the furfural yield began to decrease due to side reactions. Figure 2b shows the effect of residence time on yield, furfural selectivity, and xylose conversion. Furfural yield increased and reached a maximum at 3 h, and then the furfural yield decreased above 3 h. Furfural selectivity decreased slightly with increasing of residence time.
In the initial stage of the reaction (1–3 h), when furfural was removed from the aqueous phase to an organic phase by an extraction agent, side reactions in aqueous phase were minimized, so the furfural selectivity and yield were high. Furfural selectivity and furfural yield decreased above 3 h, and this was attributed to the longer residence time (O’Neill et al. 2009; Sievers et al. 2009).
Figure 3 shows the effect of xylose concentration on furfural yield, furfural selectivity, and xylose conversion. At a concentration of xylose higher than 10 wt.%, the xylose conversion, furfural selectivity, and furfural yield decreased with increasing xylose concentration. This was attributed to two reasons: (1) the low ratio of [H]+/[xylose] and (2) the increase of side reaction rates.
The pathway of xylose dehydration was simplified for convenient discussion (see Scheme 2). The following kinetic model was applied to describe the dehydration of xylose to furfural in ethyl butyrate/HTW.
The above reaction was assumed to be an irreversible n-order reaction. The chemical reaction rate equation can be expressed in Eq. (5).
where k, C, and n are the kinetic rate constant, xylose concentration, and the reaction order, respectively.
Jing and LÜ (2007) reported that the dehydration of xylose in HTLW (High-temperature liquid water) followed first-order kinetics. The result of this work (see Fig. 4) was different from their result. The trial method was used in this work to compute the kinetic order, and the kinetic order of the decomposition of xylose in ethyl butyrate/HTW was 0.5.
Figure 2a shows the conversion as the function of time at temperatures 160, 180, 190, and 200 °C. The dehydration rate constant (k) is obtained by Eq. (7). The kinetic parameter of xylose decomposition was estimated and the results are listed in Table 2. The reaction rate constant increased with temperature. The coefficient of determination (R 2 value) was 99.89 %.
We calculated the apparent activation energy and pre-exponential factor (Table 2) via Arrhenius plots (see Fig. 5). The estimated activation energy was distinct from the reported values (123.27 kJ/mol) (Jing and LÜ 2007) in the high-temperature water system. The extraction agent affected values of the activation energy and the pre-exponential factor.
4 Conclusions
Factors influencing dehydration of xylose to furfural were investigated. Results indicated that HTW may be used to catalyze dehydration of xylose to furfural, and ethyl butyrate had the highest extraction efficiency and improved furfural yield. The effect of temperature on furfural yield was attributed to the change of water properties at high temperatures. The furfural yield was 75 % under optimal conditions. The rate constant k showed a clear agreement with the Arrhenius law from 160 to 200 °C. The activation energy for the reaction was 68.5 kJ/mol, and the pre-exponential factor was 1.82 × 105 (mol dm−3)0.5 min−1.
References
Agirrezabal-Telleria I, Requies J, Guemez MB, et al. Dehydration of d-xylose to furfural using selective and hydrothermally stable arenesulfonic SBA-15 catalysts. Appl Catal B. 2012a;145:34–42.
Agirrezabal-Telleria I, Requies J, Guemez MB, et al. Pore size tuning of functionalized SBA-15 catalysts for the selective production of furfural from xylose. Appl Catal B. 2012b;115–116:169–78.
Akiya N, Savage PE. Roles of water for chemical reactions in high-temperature water. Chem Rev. 2002;102(8):2725–50.
Antunes MM, Lima S, Fernandes A, et al. Aqueous-phase dehydration of xylose to furfural in the presence of MCM-22 and ITQ-2 solid acid catalysts. Appl Catal A. 2012;417–418:243–52.
Chheda JN, Dumesic JA. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal Today. 2007;123(1–4):59–70.
Chheda JN, Roman-Leshkov Y, Dumesic JA. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007;9(4):342–50.
Climent MJ, Corma A, Iborra S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011;13(3):520–40.
Dias AS, Lima S, Pillinger M, et al. Acidic cesium salts of 12-tungstophosphoric acid as catalysts for the dehydration of xylose into furfural. Carbohydr Res. 2006;341(18):2946–53.
Dias AS, Pillinger M, Valente AA. Dehydration of xylose into furfural over micro-mesoporous sulfonic acid catalysts. J Catal. 2005a;229(2):414–23.
Dias AS, Pillinger M, Valente AA. Liquid phase dehydration of d-xylose in the presence of Keggin-type heteropolyacids. Appl Catal A. 2005b;285(1–2):126–31.
Erdem B, Erdem S, Öksüzoğlu RM, et al. High-surface-area SBA-15–SO3H with enhanced catalytic activity by the addition of poly(ethylene glycol). J Porous Mater. 2013;20(5):1041–9.
Hua D, Li P, Wu Y, et al. Preparation of solid acid catalyst packing AAO/SBA-15-SO3H and application for dehydration of xylose to furfural. J Ind Eng Chem. 2013;19(4):1395–9.
Jing Q, LÜ X. Kinetics of non-catalyzed decomposition of d-xylose in high temperature liquid water. Chin J Chem Eng. 2007;15(5):666–9.
Kim SB, Lee MR, Park ED, et al. Kinetic study of the dehydration of d-xylose in high temperature water. React Kinet Mech Catal. 2011;103(2):267–77.
Li G, Li N, Yang J, et al. Synthesis of renewable diesel range alkanes by hydrodeoxygenation of furans over Ni/Hβ under mild condition. Green Chem. 2014;16:594–9.
Li H, Ren J, Zhong L, et al. Production of furfural from xylose, water-insoluble hemicelluloses and water-soluble fraction of corncob via a tin-loaded montmorillonite solid acid catalyst. Bioresour Technol. 2015;176:242–8.
Lichtenthaler FW. Towards improving the utility of ketoses as organic raw materials. Carbohydr Res. 1998;313(2):69–89.
Marcotullio G, De Jong W. Chloride ions enhance furfural formation from d-xylose in dilute aqueous acidic solutions. Green Chem. 2010;12(10):1739–46.
Moreau C, Durand R, Razigade S, et al. Dehydration of fructose to 5-hydroxymethylfurfural over H-mordenites. Appl Catal A. 1996;145(1–2):211–24.
Morinelly JE, Jensen JR, Browne M, et al. Kinetic characterization of xylose monomer and oligomer concentrations during dilute acid pretreatment of lignocellulosic biomass from forests and switchgrass. Ind Eng Chem Res. 2009;48(22):9877–84.
O’Neill R, Ahmad MN, Vanoye L, et al. Kinetics of aqueous phase dehydration of xylose into furfural catalyzed by ZSM-5 zeolite. Ind Eng Chem Res. 2009;48(9):4300–6.
Promdej C, Matsumura Y. Temperature effect on hydrothermal decomposition of glucose in sub- and supercritical water. Ind Eng Chem Res. 2011;50(14):8492–7.
Shi X, Wu Y, Li P, et al. Catalytic conversion of xylose to furfural over the solid acid SO4 2-/ZrO2-Al2O3/SBA-15 catalysts. Carbohydr Res. 2011a;346(4):480–7.
Shi X, Wu Y, Yi H, et al. Selective preparation of furfural from xylose over sulfonic acid functionalized mesoporous SBA-15 materials. Energies. 2011b;4(4):669–84.
Sievers C, Musin I, Marzialetti T, et al. Acid-catalyzed conversion of sugars and furfurals in an ionic-liquid phase. Chem Sus Chem. 2009;2(7):665–71.
Zhang J, Zhuang J, Lin L, et al. Conversion of d-xylose into furfural with mesoporous molecular sieve MCM-41 as catalyst and butanol as the extraction phase. Biomass Bioenergy. 2012;39:73–7.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 21376136,No. 21176142, No. 21376140, No. 21176142, and No. 21466001) and Program for Changjiang Scholars and Innovative Research Team in University (IRT13026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Xiu-Qin Zhu
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hua, DR., Wu, YL., Liu, YF. et al. Preparation of furfural and reaction kinetics of xylose dehydration to furfural in high-temperature water. Pet. Sci. 13, 167–172 (2016). https://doi.org/10.1007/s12182-015-0069-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-015-0069-y