Abstract
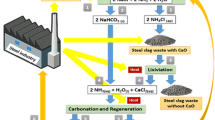
Most soda ash originates from the Solvay process that involves the aqueous reaction of NaCl, CO2, and NH3 rendering, after five steps, sodium carbonate as well as solid and liquid wastes. In the final stage, the NH4Cl originated in the first step reacts with Ca(OH)2 (lime milk), recovering most of the NH3, recycled in the process. In this work, we propose the use of steel slag, a low cost and abundant waste material of siderurgy, as source of CaO in the Solvay process. In our approach, the lime milk is partially replaced by steel slag in the NH3 recycling, recovering up to 40 wt% of the total ammonia. It can decrease the amount of CaCO3 and fossil fuels required and consequently decrease the amount of wastes originated in the procedure. In addition, we found that a simple thermal treatment of the mother liquor, immediately before the reaction with Ca(OH)2, could recover about 17–26 wt% of the NH3, reducing reagent demands and consequently effluent amounts. Another advantage is the lesser Ca(II) detected in the waste waters and the fewer caustic sludge produced in this new process. Despite it is a preliminary work, at the laboratory scale, our procedure suggests a synergic connection of the steel and soda ash productions that might reduce energy and costs, as well as the carbon dioxide emissions related to both the Na2CO3 and steel industries.
Graphical Abstract








Similar content being viewed by others
References
Alnouri SY, Linke P, El-Halwagi MM (2014) Water integration in industrial zones: a spatial representation with direct recycle applications. Clean Technol Environ Policy 16:1637–1659
Bonenfant D, Kharoune L, Sauve S, Hausler R, Niquette P, Mimeault M, Kharoune M (2008) CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind Eng Chem Res 47:7610–7616
Chiang PC, Pan SY, Chen YH, Chen CH, Chang EE (2011) Performance evaluation for carbonation of steel-making slags in a slurry reactor. J Hazard Mater 186:558–564
Eloneva S, Teir S, Salminen J, Fogelholm CJ, Zevenhoven R (2008) Steel converter slag as a raw material for precipitation of pure calcium carbonate. Ind Eng Chem Res 47:7104–7111
FHWA (2015) http://www.fhwa.dot.gov/pavement/recycling/recsslag.cfm. Accessed 28 Jan 2015
Forster M (2012) Investigations for the environmentally friendly production of Na2CO3 and HCl from exhaust CO2, NaCl and H2O. J Clean Prod 23:195–208
Gao C, Dong Y, Zhang H, Zhang J (2007) Utilization of distiller waste and residual mother liquor to prepare precipitated calcium carbonate. J Clean Prod 15:1419–1425
Gutiérrez-Arriaga CG, Abdelhady F, Bamufleh HS, Serna-González M, El-Halwagi MM, Ponce-Ortega JM (2015) Industrial waste heat recovery and cogeneration involving organic Rankine cycles. Clean Technol Environ Policy 17:767–779
Halmann M, Steinfeld A (2003) Thermoneutral coproduction of calcium oxide and syngas by combined decomposition of calcium carbonate and partial oxidation/CO2-reforming of methane. Energy Fuels 17:774–778
IEA (2013) Global action to advance carbon capture and storage: a focus on industrial applications. IEA Publications, Paris
Infomine (2015) www.infomine.com/investment/metal-prices/coal/. Accessed 28 Jan 2015
Jing Z, Liu Gd, Hang G, Lian L, Shi-huai D (2013) A theoretical basis for the relationship between the industrial pollutant generation, abatement, emission and economy. Clean Technol Environ Policy 15:707–711
Kasikowski T, Buczkowski R, Lemanowska E (2004) Cleaner production in the ammonia–soda industry: an ecological and economic study. J Environ Manag 73:339–356
Kodama S, Nishimoto T, Yamamoto N, Yogo K, Yamada K (2008) Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 33:776–784
Li W, Cui Z, Han F (2015) Methods for assessing the energy-saving efficiency of industrial symbiosis in industrial parks. Environ Sci Pollut Res 22:275–285
Murillo-Alvarado PE, Ponce-Ortega JM, El-Halwagi MM, Segovia-Hernandez JG (2013) Optimal integration of gaseous emissions from new industrial plants with the surroundings. Clean Technol Environ Policy 15:93–110
Shreve RN (1945) The chemical process industries. McGraw-Hill Book Company, São Paulo, pp 276–278
Steinhauser G (2008) Cleaner production in the Solvay Process: general strategies and recent developments. J Clean Prod 16:833–841
Trypuc M, Bialowicz K (2011) CaCO3 production using liquid waste from Solvay method. J Clean Prod 19:751–756
Trypuc M, Druzynski S (2007) Investigation of the solubility in the NaVO3–NaNO3–H2O system. Ind Eng Chem Res 46:2688–2692
Trypuc M, Lyjak G (2001) Application of NaVO3 for the utilization of the after-filtration liquor from Solvay’s process. Ind Eng Chem Res 40:2188–2191
Vogel AI (1981) Química analítica qualitativa. Mestre Jou, São Paulo, pp 330–331
Zhang H, Wang H, Zhu X, Qiu YJ, Li K, Chen R, Liao Q (2013) A review of waste heat recovery technologies towards molten slag in steel industry. Appl Energy 112:956–966
Acknowledgments
This work was supported by CNPq and FAPEMIG—Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Carvalho Pinto, P.C., de Oliveira Carvalho, M.M., Linhares, F.M. et al. A cleaner production of sodium hydrogen carbonate: partial replacement of lime by steel slag milk in the ammonia recovery step of the Solvay process. Clean Techn Environ Policy 17, 2311–2321 (2015). https://doi.org/10.1007/s10098-015-0973-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-015-0973-2