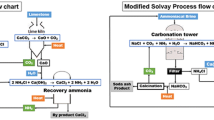
Abstract
In this work we propose the transformation of CO2 into calcium carbonate utilizing steel slag and the waste heat generated in the steel industry. The necessary chemicals, aqueous NH4Cl and solid NaHCO3, were obtained as products of a bench scale Solvay process. Our approach is divided into four steps: (i) CO2 capture using ammoniated brine, (ii) Ca2+ lixiviation from steel slag, through the reaction with NH4Cl(aq), (iii) CaCO3 precipitation by reacting the leachate with NaHCO3, and (iv) NaCl and NH3 reclamation. Steel slag is utilized as the source of calcium. A small amount of heat is required by the overall process, which could be also provided by waste heat from the steel industry. Laboratory scale experiments showed that nearly 95 wt% of NaCl and NH3 necessary for the mineral carbonation can be regenerated, therefore minimizing costs. At the end of this process, 98 wt% pure CaCO3 is obtained, and up to 94 wt% of the extracted Ca2+ was precipitated with no need for pH adjustments. Finally, we observed that, depending on the source of the steel slag, 86 kg of high purity CaCO3 could be obtained from 38 kg of CO2 and 1000 kg of steel slag.
Graphical Abstract












Similar content being viewed by others
References
Alnouri SY, Linke P, El-Halwagi MM (2014) Water integration in industrial zones: a spatial representation with direct recycle applications. Clean Technol Environ Policy 16:1637–1659
Azdarpour A, Asadullah M, Mohammadian E et al (2015) A review on carbon dioxide mineral carbonation through pH-swing process. Chem Eng J 279:615–630
Bak C, Asif M, Kim W (2015) Experimental study on CO2 capture by chilled ammonia process. Chem Eng J 265:1–8
Baldyga J, Henczka M, Sokolnicka K (2011) Mineral carbonation accelerated by dicarboxylic acids as a disposal process of carbon dioxide. Chem Eng Res Des 89(9):1841–1854
Bobicki ER, Liu Q, Xu Z et al (2012) Carbon capture and storage using alkaline industrial wastes. Prog Energ Combust 38(2):302–320
Bodor M, Santos RM, Van Gerven T et al (2013) Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation—a review. Cent Eur J Eng 3(4):566–584
Bonenfant D, Kharoune L, Sauve S et al (2008) CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind Eng Chem Res 47(20):7610–7616
Bonfils B, Julcour-Lebigue C, Guyot F et al (2012) Comprehensive analysis of direct aqueous mineral carbonation using dissolution enhancing organic additives. Int J Greenh Gas Control 9:334–346
Calado V (2003) Planejamento de Experimentos usando o Statistica. Editora E-papers
Chang E, Chen C, Chen Y et al (2011) Performance evaluation for carbonation of steel-making slags in a slurry reactor. J Hazard Mater 186(1):558–564
Darde V, van Well WJ, Stenby EH et al (2011) CO2 capture using aqueous ammonia: kinetic study and process simulation. Energy Procedia 4:1443–1450
Das B, Prakash S, Reddy P et al (2007) An overview of utilization of slag and sludge from steel industries. Resour Conserv Recycl 50(1):40–57
de Carvalho Pinto P, de Oliveira Carvalho M, Linhares F et al (2015) A cleaner production of sodium hydrogen carbonate: partial replacement of lime by steel slag milk in the ammonia recovery step of the Solvay process. Clean Techn Environ Policy 17:2311–2321
Dlugogorski BZ, Balucan RD (2014) Dehydroxylation of serpentine minerals: Implications for mineral carbonation. Renew Sustain Energy Rev 31:353–367
Dri M, Sanna A, Maroto-Valer MM (2013) Dissolution of steel slag and recycled concrete aggregate in ammonium bisulphate for CO2 mineral carbonation. Fuel Process Technol 113:114–122
Eloneva S, Mannisto P, Said A et al (2011) Ammonium salt-based steelmaking slag carbonation: precipitation of CaCO3 and ammonia losses assessment. Greenh Gases Sci Technol 1(4):305–311
Eloneva S, Said A, Fogelholm C et al (2012) Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl Energy 90(1):329–334
EPE—Empresa de Pesquisa Energética (2009) Caracterização do uso da Energia no Setor Siderúrgico Brasileiro Nota Técnica DEA 02/09
Galan I, Glasser FP, Andrade C (2013) Calcium carbonate decomposition. J Therm Anal Calorim 111(2):1197–1202
Geerlings H, Zevenhoven R (2013) CO2 mineralization-bridge between storage and utilization of CO2. Annu Rev Chem Biomol Eng 4:103–117
Gielen D, Moriguchi Y (2002) CO2 in the iron and steel industry: an analysis of Japanese emission reduction potentials. Energy Policy 30(10):849–863
Gutiérrez-Arriaga CG, Abdelhady F, Bamufleh HS, Serna-González M, El-Halwagi MM, Ponce-Ortega JM (2015) Industrial waste heat recovery and cogeneration involving organic Rankine cycles. Clean Technol Environ Policy 17:767–779
Hall C, Large D, Adderley B et al (2014) Calcium leaching from waste steelmaking slag: significance of leachate chemistry and effects on slag grain mineralogy. Miner Eng 65:156–162
Han K, Ahn CK, Lee MS et al (2013) Current status and challenges of the ammonia-based CO2 capture technologies toward commercialization. Int J Greenh Gas Control 14:270–281
Han K, Ahn CK, Lee MS (2014) Performance of an ammonia-based CO2 capture pilot facility in iron and steel industry. Int J Greenh Gas Control 27:239–246
Huang H, Shi Y, Li W et al (2001) Dual alkali approaches for the capture and separation of CO2. Energy Fuels 15(2):263–268
Huijgen WJ, Comans RN (2006) Carbonation of steel slag for CO2 sequestration: leaching of products and reaction mechanisms. Environ Sci Technol 40(8):2790–2796
IEA (International Energy Agency) (2013a) Global action to advance carbon capture and storage—A focus on industrial applications
IEA (International Energy Agency) (2013b) Technology Roadmap—Carbon capture and storage
IEA (International Energy Agency) (2014) CO2 Emissions from Fuel Combustion 2014—Highlights
Jing Z, Liu Gd, Hang G, Lian L, Shi-huai D (2013) A theoretical basis for the relationship between the industrial pollutant generation, abatement, emission and economy. Clean Technol Environ Policy 15:707–711
Jo HY, Kim JH, Lee YJ et al (2012) Evaluation of factors affecting mineral carbonation of CO2 using coal fly ash in aqueous solutions under ambient conditions. Chem Eng J 183:77–87
Jones CW (2011) CO2 capture from dilute gases as a component of modern global carbon management. Annu Rev Chem Biomol Eng 2:31–52
Kasikowski T, Buczkowski R, Lemanowska E (2004) Cleaner production in the ammonia–soda industry: an ecological and economic study. J Environ Manag 73(4):339–356
Kelly K, Silcox G, Sarofim A et al (2011) An evaluation of ex situ, industrial-scale, aqueous CO2 mineralization. Int J Greenh Gas Control 5(6):1587–1595
Kim JY, Han K, Ahn CK et al (2013) Operating cost for CO2 capture process using aqueous ammonia. Energy Procedia 37:677–682
Kirchofer A, Brandt A, Krevor S et al (2012) Impact of alkalinity sources on the life-cycle energy efficiency of mineral carbonation technologies. Energy Environ Sci 5(9):8631–8641
Kirchofer A, Becker A, Brandt A et al (2013) CO2 mitigation potential of mineral carbonation with industrial alkalinity sources in the United States. Environ Sci Technol 47(13):7548–7554
Kodama S, Nishimoto T, Yamamoto N et al (2008) Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 33(5):776–784
López-Periago AM, Pacciani R, Vega LF et al (2011) Monitoring the effect of mineral precursor, fluid phase CO2–H2O composition, and stirring on CaCO3 crystallization in a supercritical-ultrasound carbonation process. Crys Growth Des 11(12):5324–5332
Ma S, Song H, Wang M et al (2013) Research on mechanism of ammonia escaping and control in the process of CO2 capture using ammonia solution. Chem Eng Res Des 91(7):1327–1334
Ma S, Chen G, Guo M et al (2014) Path analysis on CO2 resource utilization based on carbon capture using ammonia method in coal-fired power plants. Renew Sustain Energy Rev 37:687–697
Mattila H, Grigaliūnaitė I, Zevenhoven R (2012) Chemical kinetics modeling and process parameter sensitivity for precipitated calcium carbonate production from steelmaking slags. Chem Eng J 192:77–89
Montes-Hernandez G, Perez-Lopez R, Renard F et al (2009) Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J Hazard Mater 161(2):1347–1354
Morone M, Costa G, Polettini A et al (2014) Valorization of steel slag by a combined carbonation and granulation treatment. Miner Eng 59:82–90
Niu Z, Guo Y, Zeng Q et al (2013) A novel process for capturing carbon dioxide using aqueous ammonia. Fuel Process Technol 108:154–162
Olajire AA (2013) A review of mineral carbonation technology in sequestration of CO2. J Pet Sci Eng 109:364–392
Pan S, Chang E, Chiang P (2012) CO2 capture by accelerated carbonation of alkaline wastes: a review on its principles and applications. Aerosol Air Qual Res 12(5):770–791
Pan S, Chiang P, Chen Y et al (2013a) Systematic approach to determination of maximum achievable capture capacity via leaching and carbonation processes for alkaline steelmaking wastes in a rotating packed bed. Environ Sci Technol 47(23):13677–13685
Pan S, Chiang P, Chen Y et al (2013b) Ex situ CO2 capture by carbonation of steelmaking slag coupled with metalworking wastewater in a rotating packed bed. Environ Sci Technol 47(7):3308–3315
Pan S, Chiang A, Chang E et al (2015) An innovative approach to integrated carbon mineralization and waste utilization: a review. Aerosol Air Qual Res 15:1072–1091
Pardo N, Moya JA (2013) Prospective scenarios on energy efficiency and CO2 emissions in the European iron & steel industry. Energy 54:113–128
Renforth P, Washbourne C, Taylder J et al (2011) Silicate production and availability for mineral carbonation. Environ Sci Technol 45(6):2035–2041
Rhee CH, Kim JY, Han K et al (2011) Process analysis for ammonia-based CO2 capture in ironmaking industry. Energy Procedia 4:1486–1493
Said A, Mattila HP, Jarvinen M, Zevenhoven R (2013) Production of precipitated calcium carbonate (PCC) from steelmaking slag for fixation of CO2. Appl Energy 112:765–771
Said A, Mattila O, Eloneva S et al (2015) Enhancement of calcium dissolution from steel slag by ultrasound. Chem Eng Process 89:1–8
Sanna A, Hall MR, Maroto-Valer M (2012) Post-processing pathways in carbon capture and storage by mineral carbonation (CCSM) towards the introduction of carbon neutral materials. Energy Environ Sci 5(7):7781–7796
Sanna A, Uibu M, Caramanna G et al (2014) A review of mineral carbonation technologies to sequester CO2. Chem Soc Rev 43(23):8049–8080
Santos RM, Van Bouwel J, Vandevelde E et al (2013) Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: effect of process parameters on geochemical properties. Int J Greenh Gas Control 17:32–45
Shi C (2004) Steel slag—its production, processing, characteristics, and cementitious properties. J Mater Civ Eng 16:230–236
Solvay Chemicals Soda-ammonia process. http://www.solvaychemicals.com/SiteCollectionDocuments/Soda%20Ash/Solvay%20Ammonia%20based%20process.swf. Accessed 28 Jan 2015
Steinhauser G (2008) Cleaner production in the Solvay process: general strategies and recent developments. J Clean Prod 16(7):833–841
Sun Y, Yao M, Zhang J et al (2011) Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem Eng J 173(2):437–445
Tanaka K (2012) A comparison study of EU and Japan methods to assess CO2 emission reduction and energy saving in the iron and steel industry. Energy Policy 51:578–585
Tans P, Keeling R (2015) NOAA/ESRL—trends in atmospheric carbon dioxide. www.esrl.noaa.gov/gmd/ccgg/trends/
Teir S, Eloneva S, Fogelholm C et al (2007) Dissolution of steelmaking slags in acetic acid for precipitated calcium carbonate production. Energy 32(4):528–539
Tian S, Jiang J, Li K et al (2014) Performance of steel slag in carbonation–calcination looping for CO2 capture from industrial flue gas. RSC Adv 4(14):6858–6862
Trypuć M, Białowicz K (2011) CaCO3 production using liquid waste from Solvay method. J Clean Prod 19(6):751–756
Versteeg P, Rubin ES (2011) Technical and economic assessment of ammonia-based post-combustion CO2 capture. Energy Procedia 4:1957–1964
Wang X, Maroto-Valer MM (2011) Integration of CO2 capture and mineral carbonation by using recyclable ammonium salts. ChemSusChem 4(9):1291–1300
Wang K, Wang C, Lu X et al (2007) Scenario analysis on CO2 emissions reduction potential in China’s iron and steel industry. Energy Policy 35(4):2320–2335
World Steel Association (2015) Steel’s contribution to a low carbon future and climate resilient societies—world steel position paper
Wszelaka-Rylik M, Piotrowska K, Gierycz P (2015) Simulation, aggregation and thermal analysis of nanostructured calcite obtained in a controlled multiphase process. J Therm Anal Calorim 119(2):1323–1338
Yang N, Yu H, Li L et al (2014) Aqueous ammonia (NH3) based post combustion CO2 capture: a review. Oil Gas Sci Technol 69(5):931–945
Yu J, Wang K (2011) Study on characteristics of steel slag for CO2 capture. Energy Fuels 25(11):5483–5492
Yu H, Morgan S, Allport A et al (2011) Results from trialling aqueous ammonia based post combustion capture in a pilot plant at Munmorah. Energy Procedia 4:1294–1302
Zhang Y, Dawe RA (2000) Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology. Chem Geol 163(1):129–138
Zhang H, Wang H, Zhu X et al (2013) A review of waste heat recovery technologies towards molten slag in steel industry. Appl Energy 112:956–966
Acknowledgments
This work was supported by CNPq, CAPES, and FAPEMIG—Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Carvalho Pinto, P.C., da Silva, T.R., Linhares, F.M. et al. A integrated route for CO2 capture in the steel industry and its conversion into CaCO3 using fundamentals of Solvay process. Clean Techn Environ Policy 18, 1123–1139 (2016). https://doi.org/10.1007/s10098-016-1105-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-016-1105-3