Abstract
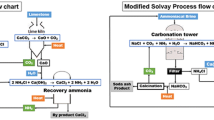
Conventional Solvay process, which utilizes CO2 to synthesize sodium carbonate, has been well-known for more than a century, but the only purpose of this process is to produce soda ash. Annually, significant amounts of CO2 are converted into soda ash using Solvay process; however, this conventional process does not sustain CO2 and waste cycle. Increasing the awareness of global warming and climate change linking to CO2 emission has pressured this industry to move for a more eco-friendly process that should exploit its potential for CO2 utilization and sequestration and thus contribute to both CO2 emission and waste management. In conventional Solvay process, to produce one mole of Na2CO3, at least one mole of CO2 might be emitted to atmosphere due to the use of CaO. Therefore, a number of studies have been done to explore novel processes or to modify the Solvay process in order for it to engage more in CO2 mitigation. This chapter introduces the most up-to-date modified Solvay process and novel pathways to produce soda ash and baking soda in the consideration of waste and CO2 utilization. Simultaneous waste and CO2 utilization offers a great opportunity for shifting to a green production, not only soda ash industry but others where their exhausted CO2, alkaline solid wastes, or reject brine can be utilized. However, there are challenges which require further research and technological development initiatives for the idea to be industrially implemented.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- C3A:
-
Ca3Al2O6
- H:
-
H2O
- AN:
-
NH4NO3
- AC:
-
NH4Cl
- AA:
-
CH3COONH4
- AS:
-
(NH4)2SO4
- MAE:
-
Methyl aminoethanol
- LDH:
-
Al layered double hydroxide
- HT:
-
Hydrotalcite-like materials
- Cl–HT:
-
Chloride-form hydrotalcite
- AOD:
-
Argon oxygen decarburization
- BOF:
-
Basic oxygen furnace
- BF:
-
Blast furnace
- EAF:
-
Electric arc furnace
- MW:
-
Municipal solid waste
- XRD:
-
X-ray diffraction
- EDS:
-
Energy dispersive X-ray spectroscopy
References
Salman M, Cizer Ö, Pontikes Y, Santos RM, Snellings R, Vandewalle L, Blanpain B, Van Balen K (2014) Effect of accelerated carbonation on AOD stainless steel slag for its valorisation as a CO2 sequestering construction material. Chem Eng J 246:39–52
Pan S-Y, Chen Y-H, Chen C-D, Shen A-L, Lin M, Chiang P-C (2015) High-gravity carbonation process for enhancing CO2 fixation and utilization exemplified by the steelmaking industry. Environ Sci Technol 49:12380–12387
Chen K-W, Pan S-Y, Chen C-T, Chen Y-H, Chiang P-C (2016) High-gravity carbonation of basic oxygen furnace slag for CO2 fixation and utilization in blended cement. J Clean Prod 124:350–360
Pan S-Y, Chung T-C, Ho C-C, Hou C-J, Chen Y-H, Chiang P-C (2017) CO2 mineralization and utilization using steel slag for establishing a waste-to-resource supply chain. Sci. Rep. 7:17227
Pei S-L, Pan S-Y, Gao X, Fang Y-K, Chiang P-C (2018) Efficacy of carbonated petroleum coke fly ash as supplementary cementitious materials in cement mortars. J Clean Prod 180:689–697
Sanna A, Dri M, Hall MR, Maroto-Valer M (2012) Waste materials for carbon capture and storage by mineralisation (CCSM)—a UK perspective. Appl Energy 99:545–554
Sanna A (2015) Reduction of CO2 emissions through waste materials recycling by mineral carbonation. Handb Clean Energy Syst
Xie H, Yue H, Zhu J, Liang B, Li C, Wang Y, Xie L, Zhou X (2015) Scientific and engineering progress in CO2 mineralization using industrial waste and natural minerals. Engineering 1:150–157
Ukwattage NL, Ranjith PG, Li X (2017) Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Measurement 97:15–22
W.S. Association (2016) Steel industry by-products, vol 2
Blissett RS, Rowson NA (2012) A review of the multi-component utilisation of coal fly ash. Fuel 97:1–23
Tayibi H, Choura M, López FA, Alguacil FJ, López-Delgado A (2009) Environmental impact and management of phosphogypsum. J Environ Manage 90:2377–2386
Saadaoui E, Ghazel N, Ben Romdhane C, Massoudi N (2017) Phosphogypsum: potential uses and problems—a review. Int J Environ Stud 74:558–567
Hammas I, Horchani-Naifer K, Férid M (2013) Solubility study and valorization of phosphogypsum salt solution. Int J Miner Process 123:87–93
Ennaciri Y, Bettach M, Cherrat A, Zegzouti A (2015) Conversion of phosphogypsum to sodium sulfate and calcium carbonate in aqueous solution. J Mater Environ Sci 7:1925
Mattila HP, Zevenhoven R (2015) Mineral carbonation of phosphogypsum waste for production of useful carbonate and sulfate salts. Front Energy Res 3
Zhao H, Li H, Bao W, Wang C, Li S, Lin W (2015) Experimental study of enhanced phosphogypsum carbonation with ammonia under increased CO2 pressure. J CO2 Utilization 11:10–19
Romero-Hermida I, Santos A, Pérez-López R, García-Tenorio R, Esquivias L, Morales-Flórez V (2017) New method for carbon dioxide mineralization based on phosphogypsum and aluminium-rich industrial wastes resulting in valuable carbonated by-products. J CO2 Utilization 18:15–22
Cárdenas-Escudero C, Morales-Flórez V, Pérez-López R, Santos A, Esquivias L (2011) Procedure to use phosphogypsum industrial waste for mineral CO2 sequestration. J Hazard Mater 196:431–435
Azdarpour A, Asadullah M, Junin R, Manan M, Hamidi H, Mohammadian E (2014) Direct carbonation of red gypsum to produce solid carbonates. Fuel Process Technol 126:429–434
Bao W, Zhao H, Li H, Li S, Lin W (2017) Process simulation of mineral carbonation of phosphogypsum with ammonia under increased CO2 pressure. J CO2 Utilization 17:125–136
Kandil A-HT, Cheira MF, Gado HS, Soliman MH, Akl HM (2017) Ammonium sulfate preparation from phosphogypsum waste. J Radiat Res Appl Sci 10:24–33
Abbas KK (2011) Study on the production of ammonium sulfate fertilizer from phosphogypsum. Eng Technol J 29:814
Chou M-IM, Bruinius JA, Benig V, Chou S-FJ, Carty RH (2005) Producing ammonium sulfate from flue gas desulfurization by-products. Energy Sour 27:1061–1071
Msila X, Billing DG, Barnard W (2016) Capture and storage of CO2 into waste phosphogypsum: the modified Merseburg process. Clean Technol Environ Policy 18:2709–2715
Ahmed M, Shayya WH, Hoey D, Mahendran A, Morris R, Al-Handaly J (2000) Use of evaporation ponds for brine disposal in desalination plants. Desalination 130:155–168
Voutchkov N (2011) Overview of seawater concentrate disposal alternatives. Desalination 273:205–219
Kaplan R, Mamrosh D, Salih HH, Dastgheib SA (2017) Assessment of desalination technologies for treatment of a highly saline brine from a potential CO2 storage site. Desalination 404:87–101
Krishnaveni V, Palanivelu K (2013) Recovery of sodium bicarbonate from textile dye bath effluent using carbon dioxide gas. Ind Eng Chem Res 52:16922–16928
Greenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P (2009) Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res 43:2317–2348
Morillo J, Usero J, Rosado D, El Bakouri H, Riaza A, Bernaola F-J (2014) Comparative study of brine management technologies for desalination plants. Desalination 336:32–49
Joo SH, Tansel B (2015) Novel technologies for reverse osmosis concentrate treatment: a review. J Environ Manage 150:322–335
Dawoud MA (2012) Environmental impacts of seawater desalination: Arabian Gulf case study
Ahmed M, Shayya WH, Hoey D, Al-Handaly J (2001) Brine disposal from reverse osmosis desalination plants in Oman and the United Arab Emirates. Desalination 133:135–147
Pérez-González A, Urtiaga AM, Ibáñez R, Ortiz I (2012) State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res 46:267–283
Giwa A, Dufour V, Al Marzooqi F, Al Kaabi M, Hasan SW (2017) Brine management methods: recent innovations and current status. Desalination 407:1–23
Ahmad N, Baddour RE (2014) A review of sources, effects, disposal methods, and regulations of brine into marine environments. Ocean Coast Manag 87:1–7
Dziedzic D, Gross KB, Gorski RA, Johnson JT (2006) Feasibility study of using brine for carbon dioxide capture and storage from fixed sources. J Air Waste Manag Assoc 56:1631–1641
Bang J-H, Yoo Y, Lee S-W, Song K, Chae S (2017) CO2 mineralization using brine discharged from a seawater desalination plant. Minerals 7:207
Breunig HM, Birkholzer JT, Borgia A, Oldenburg CM, Price PN, McKone TE (2013) Regional evaluation of brine management for geologic carbon sequestration. Int J Greenhouse Gas Control 14:39–48
El-Naas MH, Al-Marzouqi AH, Chaalal O (2010) A combined approach for the management of desalination reject brine and capture of CO2. Desalination 251:70–74
Vito CD, Mignardi S, Ferrini V, Martin RF (2011) Reject brines from desalination as possible sources for environmental technologies. In: Ning RY (ed) Expanding issues in desalination
Druckenmiller ML, Maroto-Valer MM (2005) Carbon sequestration using brine of adjusted pH to form mineral carbonates. Fuel Process Technol 86:1599–1614
Soong Y, Fauth DL, Howard BH, Jones JR, Harrison DK, Goodman AL, Gray ML, Frommell EA (2006) CO2 sequestration with brine solution and fly ashes. Energy Convers Manag 47:1676–1685
Zhao Y, Zhang Y, Liu J, Gao J, Ji Z, Guo X, Liu J, Yuan J (2017) Trash to treasure: seawater pretreatment by CO2 mineral carbonation using brine pretreatment waste of soda ash plant as alkali source. Desalination 407:85–92
Wang W, Hu M, Zheng Y, Wang P, Ma C (2011) CO2 Fixation in Ca2+/Mg2+ rich aqueous solutions through enhanced carbonate precipitation. Ind Eng Chem Res 50:8333–8339
Wagialla KM, Al-Mutaz IS, El-Dahshan ME (1992) The manufacture of soda ash in the Arabian Gulf. Int J Prod Econ 27:145–153
Steinhauser G (2008) Cleaner production in the Solvay process: general strategies and recent developments. J Clean Prod 16:833–841
Jadeja RN, Tewari A (2009) Effect of soda ash industry effluent on agarophytes, alginophytes and carrageenophyte of west coast of India. J Hazard Mater 162:498–502
Kasikowski T, Buczkowski R, Cichosz M, Lemanowska E (2007) Combined distiller waste utilisation and combustion gases desulphurisation method: the case study of soda-ash industry. Resour Conserv Recycl 51:665–690
Kasikowski T, Buczkowski R, Cichosz M (2008) Utilisation of synthetic soda-ash industry by-products. Int J Prod Econ 112:971–984
Kasikowski T, Buczkowski R, Dejewska B, Peszyńska-Białczyk K, Lemanowska E, Igliński B (2004) Utilization of distiller waste from ammonia-soda processing. J Clean Prod 12:759–769
Kasikowski T, Buczkowski R, Lemanowska E (2004) Cleaner production in the ammonia–soda industry: an ecological and economic study. J Environ Manage 73:339–356
de Carvalho Pinto PC, da Silva TR, Linhares FM, de Andrade FV, de Oliveira Carvalho MM, de Lima GM (2016) A integrated route for CO2 capture in the steel industry and its conversion into CaCO3 using fundamentals of Solvay process. Clean Technol Environ Policy 18:1123–1139
Trypuć M, Białowicz K (2011) CaCO3 production using liquid waste from Solvay method. J Clean Prod 19:751–756
Shan Y, Liu Z, Guan D (2016) CO2 emissions from China’s lime industry. Appl Energy 166:245–252
Hemalatha T, Ramaswamy A (2017) A review on fly ash characteristics—towards promoting high volume utilization in developing sustainable concrete. J Clean Prod 147:546–559
Pan S-Y, Chang EE, Chiang P-C (2012) CO2 Capture by accelerated carbonation of alkaline wastes: a review on its principles and applications. Aerosol Air Qual Res 12:770–791
Eloneva S, Teir S, Salminen J, Fogelholm C-J, Zevenhoven R (2008) Steel converter slag as a raw material for precipitation of pure calcium carbonate. Ind Eng Chem Res 47:7104–7111
Jo H, Park S-H, Jang Y-N, Chae S-C, Lee P-K, Jo HY (2014) Metal extraction and indirect mineral carbonation of waste cement material using ammonium salt solutions. Chem Eng J 254:313–323
Said A, Mattila H-P, Järvinen M, Zevenhoven R (2013) Production of precipitated calcium carbonate (PCC) from steelmaking slag for fixation of CO2. Appl Energy 112:765–771
He L, Yu D, Lv W, Wu J, Xu M (2013) A novel method for CO2 sequestration via indirect carbonation of coal fly ash. Ind Eng Chem Res 52:15138–15145
de Carvalho Pinto PC, de Oliveira Carvalho MM, Linhares FM, da Silva TR, de Lima GM (2015) A cleaner production of sodium hydrogen carbonate: partial replacement of lime by steel slag milk in the ammonia recovery step of the Solvay process. Clean Technol Environ Policy 17:2311–2321
Sun Y, Yao M-S, Zhang J-P, Yang G (2011) Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem Eng J 173:437–445
Said A, Laukkanen T, Järvinen M (2016) Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl Energy 177:602–611
Yong S, Ping ZJ, Lian Z (2016) NH4Cl selective leaching of basic oxygen furnace slag: optimization study using response surface methodology. Environ Progress Sustain Energy 35:1387–1394
Sanni E, Sebastian T, Hannu R, Justin S, Arshe S, Carl-Johan F, Ron Z (2009) Reduction of CO2 emissions from steel plants by using steelmaking slags for production of marketable calcium carbonate. Steel Res Int 80:415–421
Hall C, Large DJ, Adderley B, West HM (2014) Calcium leaching from waste steelmaking slag: significance of leachate chemistry and effects on slag grain mineralogy. Miner Eng 65:156–162
Hosseini T, Selomulya C, Haque N, Zhang L (2014) Indirect carbonation of victorian brown coal fly ash for CO2 sequestration: multiple-cycle leaching-carbonation and magnesium leaching kinetic modeling. Energy Fuels 28:6481–6493
Lee SM, Lee SH, Jeong SK, Youn MH, Nguyen DD, Chang SW, Kim SS (2017) Calcium extraction from steelmaking slag and production of precipitated calcium carbonate from calcium oxide for carbon dioxide fixation. J Ind Eng Chem 53:233–240
Lee S, Kim JW, Chae S, Bang JH, Lee SW (2016) CO2 sequestration technology through mineral carbonation: An extraction and carbonation of blast slag. J CO2 Utilization 16:336–345
Tong Z, Ma G, Zhang X, Cai Y (2017) Microwave-supported leaching of electric arc furnace (EAF) slag by ammonium Salts. Minerals 7:119
Mattila HP, Zevenhoven R (2014) Chapter ten—production of precipitated calcium carbonate from steel converter slag and other calcium-containing industrial wastes and residues. In: Aresta M, van Eldik R (eds) Advances in inorganic chemistry. Academic Press, pp 347–384
Sun Y, Parikh V, Zhang L (2012) Sequestration of carbon dioxide by indirect mineralization using victorian brown coal fly ash. J Hazard Mater 209–210:458–466
Jo H, Lee M-G, Park J, Jung K-D (2017) Preparation of high-purity nano-CaCO3 from steel slag. Energy 120:884–894
Park HK, Bae MW, Nam IH, Kim S-G (2013) Acid leaching of CaOSiO2 resources. J Ind Eng Chem 19:633–639
Solvay E (1882) Manufacture of soda by the ammonia process. USA
El Naas M (2011) Reject brine management, desalination, trends and technologies. InTech, Croatia, pp 237–252
El-Naas M, Ali A-M, Omar C (2010) A combined approach for the management of desalination reject brine and capture of CO2. Desalination 251:70–74
Joris K, Andrea R, Toon VH, Arjan VH, Wim T, Andre F (2010) The impact of CO2 capture in the power and heat sector on the emission of SO2, NOx, particulate matter, volatile organic compounds and NH3 in the European Union. Atmos Environ 44:1369–1385
Dave N, Do T, Puxty G, Rowland R, Feron PHM, Attalla MI (2009) CO2 capture by aqueous amines and aqueous ammonia—a comparison. Energy Procedia 1:949–954
Huang HP, Shi Y, Li W, Chang SG (2001) Dual alkali approaches for the capture and separation of CO2. Energy Fuels 15:263–268
Dindi A, Quang DV, Abu-Zahra MR (2015) Simultaneous carbon dioxide capture and utilization using thermal desalination reject brine. Appl Energy 154:298–308
Abdel-Aal HK, Ibrahim AA, Shalabi MA, Al-Harbi DK (1996) Chemical separation process for highly saline water 1. Parametric Exp Invest Ind Eng Chem Res 35:799–804
Abdel-Aal H, Ibrahim A, Shalabi M, Al-Harbi D (1997) Dual-purpose chemical desalination process. Desalination 113:19–25
Dindi A, Quang DV, El Hadri N, Rayer A, Abdulkadir A, Abu-Zahra MR (2014) Potential for the simultaneous capture and utilization of CO2 using desalination reject brine: amine solvent selection and evaluation. Energy Procedia 63:7947–7953
Vaidya PD, Kenig EY (2007) CO2-alkanolamine reaction kinetics: a review of recent studies. Chem Eng Technol 30:1467–1474
Conway W, Wang X, Fernandes D, Burns R, Lawrance G, Puxty G, Maeder M (2013) Toward the understanding of chemical absorption processes for post-combustion capture of carbon dioxide: electronic and steric considerations from the kinetics of reactions of CO2 (aq) with sterically hindered Amines. Environ Sci Technol 47:1163–1169
El-Naas MH, Mohammad AF, Suleiman MI, Al Musharfy M, Al-Marzouqi AH (2017) A new process for the capture of CO2 and reduction of water salinity. Desalination 411:69–75
Dindi A, Quang DV, AlNashef I, Abu-Zahra MR (2018) A process for combined CO2 utilization and treatment of desalination reject brine. Desalination 442:62–74
Theiss FL, Sear-Hall MJ, Palmer SJ, Frost RL (2012) Zinc aluminium layered double hydroxides for the removal of iodine and iodide from aqueous solutions. Desalin Water Treat 39:166–175
Nenoff TM, Sasan K, Brady PV, Krumhansl JL, Paap S, Heimer B, Howe K, Stoll Z, Stomp J (2017) Waste water for power generation via energy efficient selective silica separations. Sandia National Lab. (SNL-NM), Albuquerque, NM (United States)
Kameda T, Yabuuchi F, Yoshioka T, Uchida M, Okuwaki A (2003) New method of treating dilute mineral acids using magnesium–aluminum oxide. Water Res 37:1545–1550
Setshedi K, Ren J, Aoyi O, Onyango MS (2012) Removal of Pb (II) from aqueous solution using hydrotalcite-like nanostructured material. Int J Phys Sci 7:63–72
Douglas G, Wendling L, Pleysier R, Trefry M (2010) Hydrotalcite formation for contaminant removal from Ranger mine process water. Mine Water Environ 29:108–115
Kameda T, Yoshioka T, Hoshi T, Uchida M, Okuwaki A (2005) The removal of chloride from solutions with various cations using magnesium–aluminum oxide. Sep Purif Technol 42:25–29
Kameda T, Oba J, Yoshioka T (2017) Simultaneous removal of Cl− and SO42− from seawater using Mg–Al oxide: kinetics and equilibrium studies. Appl Water Sci 7:129–136
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Quang, D.V., Dindi, A., Abu Zahra, M.R.M. (2019). The Utilization of CO2, Alkaline Solid Waste, and Desalination Reject Brine in Soda Ash Production. In: Winter, F., Agarwal, R., Hrdlicka, J., Varjani, S. (eds) CO2 Separation, Purification and Conversion to Chemicals and Fuels. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-13-3296-8_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-3296-8_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3295-1
Online ISBN: 978-981-13-3296-8
eBook Packages: EnergyEnergy (R0)