Abstract
Bacteria have their own language through which they communicate with one another like all higher organisms. So, many researchers are working hard to identify and comprehend the components of this bacterial communication, known as quorum sensing (QS). In quorum sensing, bacteria use signaling molecules called autoinducers (AIs) to exchange information. Many natural compounds and extraction techniques have been intensively studied to disrupt bacterial signaling and examine their effectiveness for bacterial pathogenesis control. Quorum sensing inhibitors can interfere with QS and block the action of AI signaling molecules. Recent research indicates that quorum sensing inhibitors (QSIs) and quorum quenching enzymes (QQEs) show great promise in reducing the pathogenicity of bacteria and inhibiting biofilm synthesis. In addition, the effectiveness of QQEs and QSIs in experimental animal models was demonstrated. These are taken into account in the development of innovative medical devices, such as dressings and catheters, to prevent bacterial infections. The present review highlights this aspect with a prospective vision for its development and application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
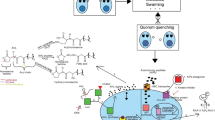
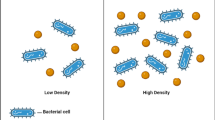
Quorum sensing (QS) is the process of bacterial cell-to-cell communication that utilizes their activities to act together in a group manner [1]. At high cell density, on reaching the threshold level, QS allows bacteria to perform processes like biofilm formation, virulence factor synthesis, siderophore production, and enzyme production [2]. QS is characterized by the production of small signaling molecules called autoinducers (AIs), which are coordinated by cell density [3]. In Gram-positive bacteria such as Staphylococcus spp., autoinducing peptides (AIPs) have been shown to promote QS [4]. While acyl-homoserine lactones (AHLs) were found to be the AIs in Gram-negative bacteria such as Acinetobacter spp., Pseudomonas spp., and Burkholderia spp. AHLs are made up of an acyl chain and a lactone ring that varies in structure, length, and cellular activity Fig. 1 [5].
There are many other AIs that have also been identified, such as ketones which are used by Legionella spp. and Vibrio cholera spp. [6] and fatty acids that are utilized by Burkholderia spp. and Xanthomonas spp [7]. Autoinducer-2 (AI-2) is utilized by Gram-positive and Gram-negative bacteria Fig. 1 [8]. Some Gram-negative bacteria use multiple QS systems, either in parallel, like in Vibrio harveyi, where three systems are linked together to form a single regulatory cascade Fig. 2. The first system synthase is LuxM, which produces the AI; MAI-1, the second is LuxS, which produces AI-2, and the third is CqsA, which produces CAI-1. Others, such as P. aeruginosa, employ a hierarchical cascade with four QS systems. The first system is named LasI/LasR, the second is RhlI/RhlR, the third is PqsABCDE/PqsR, and the fourth is AmbBCDE/IqsR system [9, 10]. Each system employs a different AI; 3-oxododecanoyl-L-homoserine lactone (3-oxo-C12-HSL), N-butanoyl homoserine lactone (C4-HSL), 2-heptyl-3-hydroxy-4-quinolone (Pseudomonas quinolone signal, PQS), and 2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde, respectively Fig. 3.
Elimination of quorum sensing
There are a number of methods that can disrupt or quench bacterial communication, including: (i) using quorum sensing inhibitors (QSIs) for the interrupting of AIs [3, 11, 12] (ii) AIs scavenging by using macromolecules, antibodies of quorum quenching [13], or (iii) using QQ enzymes (QQEs) to hydrolyze the AIs extracellularly [14]. There are numerous natural compounds that can act as QSIs. For instance, polyphenols that were isolated from beans, carrots, chamomile [15], and Trigonella stellata [16]. Some other QSIs may be synthetic, such as aspirin [17], β-lactams [18], azithromycin [19], and benzothiazole derivatives[20].
Many QQEs were reported to disrupt QS in Gram-negative bacteria and degrade AHL. For example, the acylase enzyme breaks down the amide linkage between the side chain of fatty acid and lactone ring [21]. The acylase enzyme was isolated from Ochrobactrum sp. [22], P. aeruginosa [23], and Streptomyces sp. [24]. Also, lactonase enzymes that hydrolyze the lactone ring were isolated from Bacillus sp. [25], Klebsiella pneumoniae, Arthrobacter sp. [26], and Rhodococcus erythropolis [27]. The third family of known AHL QQEs are the oxidoreductases. This group of enzymes acts by oxidizing or reducing the acyl chains of the AHLs rather than destroying them [3]. These processes do not result in signal degradation, but they modify the specificity, which has an impact on the interaction between the signal and the receptor Fig. 4. Paraoxonases enzymes (PONs) are another group of human enzymes that have recently been identified as QQ agents. PONs are capable of AHL-mediated QS disruption as acylase and lactonase enzymes [28]. PONs are thought to play a significant role in innate human defense mechanisms that inactivate harmful P. aeruginosa AHL signals [29].
QQ approaches have applications in a variety of fields like agriculture, water treatment, fisheries and aquaculture, and health care [3, 30]. Recently, considerable attention has been paid to finding novel therapeutic approaches with the increasing antibiotic resistance [31, 32].
The relationship between antimicrobial resistance and QS
Several studies reported that there is a correlation between QS and antibiotic resistance because QS is a crucial regulator of biofilm development. As an instance, the incorporation of AHLs into a culture of P. aeruginosa increased resistance against ciprofloxacin and carbenicillin antibiotics [33]. Also, a transcriptomic study in P. aeruginosa PA14 showed that QS increased PqsR expression, which protects against β-lactam antibiotics and H2O2 [34]. The global regulator VqsM, which promotes QS, was identified as a major contributor to antibiotic resistance against quinolones, kanamycin, and tetracycline [35]. The physiological factors play a role in QS-mediated antibiotic tolerance, but many studies have emphasized the significance of biofilm in bacterial antibiotic resistance [36]. Biofilm is a heterogeneous multicellular structure attached to a solid substrate [37]. The extracellular matrix (ECM) of biofilms, composed of extracellular DNA, polysaccharides, and proteins that plays a crucial role in enhancing antibiotic resistance. This resistance is attributed to the protective ECM barrier, slower bacterial growth rates within the biofilm, and the presence of persisted cells [38]. This biofilm also can significantly enhance the bacterial pathogenicity through several mechanisms like enhancing the bacterial communication and coordination via QS, this allows bacteria to coordinate their behavior, including the regulation of virulence factor production [39, 40]. They protect bacteria from immune responses by acting as a physical barrier, preventing phagocytosis and neutralizing antibodies [41]. The close proximity of bacterial cells within a biofilm facilitates horizontal gene transfer, spreading antibiotic resistance genes and virulence factors, thereby increasing pathogenic potential [42]. Biofilms contribute to persistent infections, as they are difficult to eradicate and can lead to chronic and recurrent infections. Effective communication through quorum sensing within biofilms regulates virulence genes and coordinates toxin release [43]. Additionally, Biofilms provide a stable environment for bacteria, enabling survival under hostile conditions such as nutrient deprivation and pH changes [44]. As a result, bacteria within a biofilm can mount a more coordinated and effective attack on the host. Some QSIs were employed to increase the sensitivity of antibiotics and to lower the doses of antibiotics [45]. Additionally, in the mouse model, combining antimicrobial medications with QQEs as lactonase had a synergistic impact and boosted antibiotic efficiency (Fig. 5). The effectiveness of QQEs and QSIs in the treatment and prevention of infections has been approved. However, it cannot be used in alone to treat acute infections caused by antibiotic-resistant bacteria.
Quorum sensing and biofilm formation
Biofilm-forming bacteria are believed to be 1000 times more resistant to antimicrobial agents [46] and it is estimated that biofilm-associated infections account for 80%. This occurs either through infected tissues, as in cystic fibrosis, or via contaminated devices. Biofilm encourages the emergence of various phenotypes and defense mechanisms, including alterations in cellular physiological state, physical barrier formation, and gene expression. Inhibitor that disrupts QS systems will consequently alter the biofilm formation. For instance, P. aeruginosa’s Las, Rhl, and PQS QS systems are necessary for the synthesis of biofilms (Fig. 3), and any changes are associated with increased susceptibility to both antimicrobials and the human immune system [47]. Some antibiotics like levofloxacin and meropenem were found to increase the expression of efflux pumps, which boost the production of AHL in Acinetobacter baumannii clinical isolates. As a result, biofilm development increases the antibiotic resistance. As mentioned earlier, QS controls both biofilm development and antibiotic resistance. So, the use of QQ molecules was studied. Using some pharmacological agents such as benzamide-benzimidazole in P. aeruginosa could inhibit the QS regulators, reduced biofilm development, and decreased antibiotic resistance [48]. Some QSIs, such as hamamelitannin and baicalin hydrate, improved the biofilm disintegration. In vivo and in vitro, they exhibited synergistic effects against Gram-negative bacteria when QSIs were coupled with antibiotics such as tobramycin, vancomycin, or clindamycin [49]. Besides, the efficacy of a wide range of antibiotics, such as quinolones [50], cephalosporins, and glycopeptides [48] was improved when added to QSIs.
The relationship between sensitivity to bacteriophages and quorum sensing
In the last decade, there has been massive interest in using phage therapy to treat infections caused by multidrug-resistant (MDR) bacteria. Bacteriophages are the most numerous bacterial predators on Earth and are utilized for treating bacterial infections [51]. Despite the intriguing potential of bacteriophages as a solution, bacteria have developed mechanisms of resistance to evade their effects [52]. For example, bacteria can reduce phage entry by increasing extracellular matrix formation and altering the structure of phage receptors [53]. Bacteria can also recognize and degrade phage DNA with the aid of restriction enzymes [54]. The first pathogen demonstrating a correlation between bacteriophage sensitivity and quorum sensing (QS) was P. aeruginosa [55]. However, it took years to establish that QS modulates mechanisms of phage defense in Escherichia coli by reducing the chi adsorption rate and phage lambda. The absence of AI synthase genes in Vibrio cholera lowered phage resistance, which was restored after the addition of AI-2, CAI-1, and AIs [56]. Similarly, the resistance of phages increased when synthetic AHLs were added to the QS-deficient strain Vibrio anguillarum [57]. In addition, the expression of ompK, the phage receptor, decreased with AHL production, and the absence of QS signaling systems impeded both the DNA degradation process regulated by this system and the acquisition of immunity [58]. This phenomenon was observed in Burkholderia glumae and Pectobacterium atrosepticum as well. Furthermore, the RhlI and LasI QS systems in P. aeruginosa PA14 were found to control CRISPR-Cas gene expression [59]. Interestingly, doubling the number of sensitive cells in the culture using penicillic acid enhanced P. aeruginosa phage sensitivity [60]. These findings are not particularly surprising when considering that the use of quorum quenching (QQ) molecules is a highly promising strategy [3]. Indeed, incorporating phage therapy alongside QQ molecules may enhance bacteriophage susceptibility. Furthermore, in multi-microbial cultures, disrupting the quorum sensing (QS) of one species led to a decrease in overall biomass. Consequently, it can be concluded that the combined use of QQ molecules and phage treatment is effective against various microbial infections [61].
In vivo antivirulence activity of quorum quenchers
In recent years, there has been a growing interest in elucidating the in vivo antivirulence activity of quorum quenchers (QQs). To assess the significance of quorum sensing inhibitors (QSIs) and quorum quenching enzymes (QQEs) on microbial virulence and pathogenicity, researchers have developed various models. These models employ three main approaches, ranging from simple unicellular models to more intricate systems. The following section provides a summary of these approaches.
Model of amoebal infection
A model of amoebal infection has been developed to investigate the interaction between pathogens and host immune responses. The immune system responds to amoebae antigens through lysosomal digestion and phagocytosis defense mechanisms, similar to the bacterial inhibition mechanism used by macrophages [62]. Leveraging this close relationship, amoebae have been employed as a model to study the synthesis of bacterial virulence factors [63]. The ability of amoebae to thrive in the presence of pathogenic bacteria has been a valuable tool for assessing bacterial pathogenicity [64]. This approach has been utilized across various bacterial species to examine the relationship between quorum quenching (QQ) molecules and virulence factors. Recently, a QQ enzyme was evaluated using the amoeba model, based on a well-established test involving Dictyostelium discoideum and P. aeruginosa. In the plate-killing test of D. discoideum, the overproduction of P. aeruginosa PA14 with the aliphatic amidase disrupted quorum sensing (QS) and reduced pathogenicity toward the amoeba [63]. While this approach is quick and practical for studies, its application is limited due to the potential impact of amoeba species and culture conditions on result accuracy [65].
Infection model of Caenorhabditis elegans
Caenorhabditis elegans has become a predominant model for studying bacterial pathogenicity, offering valuable insights into the impact of quorum sensing (QS) on bacterial virulence [66]. Unlike amoebae, C. elegans possesses an innate immune system that closely mimics the human immune response, making it a highly relevant model for investigating pathogenicity [66]. The survival rate of C. elegans is commonly measured using specific microorganisms of interest. Numerous experimental trials, particularly with bacterial mutants deficient in autoinducer (AI) production, have been conducted to assess the significance of QS in pathogenicity. The introduction of quorum sensing inhibitors (QSIs) resulted in a reduction in worm mortality after infection with various P. aeruginosa strains, highlighting the role of QS in bacterial pathogenicity [67]. C. elegans has also been employed to explore the relationship between QS and pathogenicity in other Gram-negative bacteria, such as Yersinia pseudotuberculosis and E. coli [68]. Additionally, for Gram-positive bacteria, a connection between QS and pathogenicity has been established, as seen in S. aureus and Enterococcus faecalis [69]. In summary, these studies collectively demonstrate that QSI can effectively eliminate pathogenicity across a broad spectrum of bacteria.
C. elegans has been employed in vitro to validate the effectiveness of quorum sensing inhibitors (QSIs) and quorum quenching enzymes (QQEs). However, the impact on survival is subject to variation based on cultural conditions. For instance, the QQE BpiB09, which targets AHLs, significantly increased the survival of C. elegans, with rates reaching up to 100% [70]. Similarly, the quorum sensing inhibitor (QSI) 4-nitro-pyridine-N-oxide restored the worm’s survival completely by targeting the quorum sensing signaling system of P. aeruginosa PAO1 [15]. Natural quorum sensing inhibitor (QSI) extracts from Callistemon viminalis, Bucida buceras, and Conocarpus were shown to restore up to 87% survival [71]. Additionally, hamamelitannin and baicalin, known QSIs, have demonstrated synergistic effects with antibiotics [49].
C. elegans stands out as an especially useful invertebrate model, allowing for virtual screening of quorum quenching (QQ) compounds’ activity and studying bacterial mutations. It offers a comprehensive understanding of the regulation and modification of virulence factors by QQ [72]. QQEs and QSIs have proven effective in reducing mortality in C. elegans caused by a wide range of bacteria, showcasing the potential of QQ as an antipathogenic drug in multicellular organisms. However, certain restrictions exist, such as the living parameters of the worm, which differ from those of bacteria (e.g., the optimal growing temperature is 20 °C), and the significant physiological differences between roundworms and humans. Furthermore, like amoebae, various studies have highlighted the impact of conditions on assay results [73].
Model of murine infection
To investigate the role of quorum sensing (QS) in bacterial infections, mammalian models such as mice or rats are commonly employed. Notably, deletions or mutations of QS-related genes have been shown to significantly reduce mortality and the severity of infections, including burn wounds [74], the prostate [75], and peritonitis [69] (Fig. 5).
QQ molecules have demonstrated a capacity to reduce mortality and hasten recovery. For instance, the furanone compound decreased P. aeruginosa colonization and associated mortality in lung infections of mammalian models [76]. Similarly, garlic extract [77] yielded comparable results in reducing P. aeruginosa colonization. QQEs have also proven effective in P. aeruginosa burn infections [45] and tea polyphenol quorum sensing inhibitors (QSIs) demonstrated efficacy in injury models [78]. Likewise, a variety of QSIs inhibited the expression of virulence factors in S. aureus during skin wound infections [79].
Moreover, extensive in vivo studies have explored the effectiveness of combining antibiotics with quorum quenching (QQ) molecules against both Gram-negative and Gram-positive bacteria. For instance, the combination of tobramycin and baicalin, a quorum sensing inhibitor (QSI), significantly reduced lung colonization by B. cenocepacia [49]. In another example, the combination of ciprofloxacin with a lactonase enzyme reduced mortality and bacterial spread to internal organs in mice with P. aeruginosa burn infections [45]. Additionally, the use of QSIs in combination with antibiotics markedly decreased microbial colonization induced by both S. aureus [80] and S. epidermidis [81].
These instances have generated interest in exploring the use of quorum quenching (QQ) molecules to mitigate antibiotic resistance in organ infections. Murine models prove invaluable for studying the impact of QQ molecules on bacterial infections, given their proximity to human physiology and their possessing both innate and adaptive immune systems. Additionally, murine models are often essential as preliminary tests before advancing to human clinical trials. The application of QQ molecules appears highly effective in reducing morbidity and adverse effects across various types of infections. However, murine models are less amenable to screening procedures compared to amoeba and C. elegans models, primarily due to ethical and practical considerations [82].
Human clinical trials with quorum sensing inhibitors
The application of quorum quenching (QQ) molecules is rapidly advancing, with new inhibitors continually being discovered. Despite this progress, clinical applications are still under investigation, and only three human clinical trials on quorum sensing inhibitors (QSIs) have been conducted. The first trial utilized sub-inhibitory concentrations of the azithromycin antibiotic in the treatment of cystic fibrosis [83]. It demonstrated efficacy in vitro by inhibiting the signaling system in P. aeruginosa. Subsequently, it underwent evaluation in patients with ventilator-associated pneumonia [84]. Azithromycin’s antivirulence properties were examined in a high-risk patient group, but the findings did not reach statistical significance. The second study focused on garlic, a well-known quorum sensing inhibitor (QSI) utilized in cystic fibrosis treatment, although there is limited evidence supporting its therapeutic effect on pathogenic infections [85]. Finally, 5-fluorouracil (5-FU), an anti-cancer medicine, was discovered to inhibit virulence factors in vitro in P. aeruginosa and was applied as a coating for catheters. It demonstrated high efficacy in clinical trials [86]. Nonetheless, further research is required to confirm the therapeutic efficacy of this method in broader clinical trials.
Use of quorum quenching molecules in medical devices
Medical equipment has been implicated in various hospital-acquired infections (HAIs) [87]. These infections, often caused by multidrug-resistant (MDR) and/or biofilm-forming bacteria, pose significant medical challenges and carry a high risk of mortality. Recognizing the potential of quorum quenching (QQ) to eliminate bacterial pathogenicity, novel medical devices incorporating QQ agents have been developed. Examples include the latest generations of contact lenses [88], implantable devices [89], catheters [90], or aerosols [91]. The initial application of quorum sensing inhibitors (QSIs) was in the modification of catheter surfaces. For instance, furanone was shown to reduce the biofilm of S. epidermidis, preventing infection for two months [92]. Similarly, catheters coated with 5-fluorouracil (5-FU) reduced Gram-negative bacterial contamination levels compared to conventional coatings. A varnish-based delivery method, producing the QSI thiazolidinedione-8, demonstrated efficacy against biofilms on catheters [93]. Combinations of furanone derivatives and dihydropyrrol-2-ones on glass surfaces decreased the adherence of both P. aeruginosa PAO1 and S. aureus SA38 [94].
Surfaces were also coated with anti-QS peptides such as TrAIP-II and AIP-I which were effective against S. aureus [95]. The combination of the daptomycin antibiotic with the QS-inhibiting peptide FS3 coated prosthesis demonstrated a synergistic effect against staphylococcus infection [96].
Likewise, the addition of the RNAIII inhibiting peptide (RIP) to the Dacron graft demonstrated high efficacy in reducing S. epidermidis infection [81]. In a rabbit model, the combination of α-amylase with acylase from Bacillus amyloliquefaciens resulted in decreased biofilm growth for both E. coli and P. aeruginosa [97]. Additionally, porcine kidney acylase adsorbed on nanofibers was utilized to create biocatalysts that inhibited P. aeruginosa PAO1 biofilm formation. The topical application of lactonase from Bacillus sp. ZA12 to mice infected with P. aeruginosa PAO1 had a synergistic effect with ciprofloxacin [45]. Considering the significance of enzyme stability in developing bio-based products, extremophile catalysts were also explored. The PLL SsoPox enzyme, isolated from Sulfolobus solfataricus, emerged as a particularly promising method for quenching bacterial pathogenicity [30]. This exceptionally durable enzyme was initially immobilized into nanoalumina membranes, maintaining its high efficiency in reducing virulence factor secretions of P. aeruginosa PAO1, such as pyocyanin and elastase [98]. After immobilization in a polyurethane covering using glutaraldehyde crosslinking, the SsoPox-W263I enzyme demonstrated its ability to reduce the pathogenicity of 51 P. aeruginosa clinical isolates from diabetic foot ulcers and maintained effectiveness toward P. aeruginosa PAO1 [99]. In a rat model with chronic respiratory infection by P. aeruginosa PAO1, the use of this mutant enzyme resulted in an increased survival rate [91]. Devices based on quorum quenching (QQ) have garnered significant attention due to their potential to prevent hospital-acquired infections (HAIs) by reducing bacterial pathogenicity and inhibiting biofilm formation. However, further in vivo and clinical trials are essential to establish their efficacy. The effectiveness of these devices must be validated across different bacterial strain models, including phenotypically and genetically diverse clinical isolates. While medical technology advanced devices face less regulation than pharmaceuticals, there is still ample room for innovation. Concerns must be addressed to validate the potential application of therapeutic procedures in the project. Nonetheless, the broad scope of quorum quenching enzymes (QQEs) and quorum sensing inhibitors (QSIs), along with numerous examples of potential clinical relevance, paves the way for the development of new devices.
Conclusions and future perspectives
Over the past two decades, numerous studies have demonstrated the significant anti-pathogenic effects of QQ compounds and methods against a broad spectrum of bacteria. Various techniques for functionalizing medical equipment have shown substantial benefits. Yet, the probability of bacteria developing resistance to QQ mechanisms remains poorly understood. Resistance is the consequence of a natural evolutionary process that favors the formation of new, resistant strains. This is concerning because antibiotics significantly suppress the growth of sensitive bacteria. While some QSIs strongly inhibit growth, while others have minimal effects. QSI-resistant bacterial strains may likely develop, albeit at a slower rate than antibiotic-resistant strains. The specific QSI molecules and its impact on bacterial growth determine the emergence rate. Laboratory experiments have reported QSI-resistant bacteria, but the competitive advantage of these strains over QSI-sensitive ones remains unclear.
To mitigate QQ resistance, careful selection of QSI molecules is crucial, considering that many QSIs are toxic and require cell penetration for activation. The QQEs with potent virulence-inhibiting effects, may be promising candidates. Further studies employing QQEs are needed to identify putative QQ resistance mechanisms. The significant impact of QS on bacterial physiology suggests that QQ could not only eliminate bacterial pathogenicity but also restore antibiotic tolerance, paving the way for future therapeutic strategies. Surprisingly, disrupting bacterial signaling systems has consequences beyond the physiology of individual cells. More research is needed to understand the effects of QQ methods at both the individual bacterial and community levels. The next decade is expected to witness a significant increase in understanding QQ molecules, with widespread applications as coating agents in medical devices, potentially replacing bacteriostatic and bactericidal antibiotics. Additionally, innovative applications for QSIs include their use in combination therapies with antibiotics and phage therapy to enhance bacterial eradication, integration into agricultural practices for crop protection and livestock health, incorporation into medical device coatings to prevent biofilm formation, wound care treatments, food preservation, water treatment facilities, bioremediation efforts, engineered probiotics, oral care products, veterinary medicine, and anti-cancer strategies. These diverse applications underscore the potential of QSIs to address various challenges across multiple fields, promoting more effective and sustainable solutions. More investigations into the use of QQ molecules in human trials are anticipated.
Data availability
No datasets were generated or analysed during the current study.
References
El-Metwally MM, Mekawey AAI, El-Halmouch Y, Naga NG (2023) Symbiotic relationships with Fungi: from mutualism to Parasitism. Plant Mycobiome: diversity, interactions and uses. Springer, pp 375–413
Naga NG, El-Badan DE-S, Rateb HS et al (2021) Quorum Sensing Inhibiting Activity of Cefoperazone and its Metallic Derivatives on Pseudomonas aeruginosa Frontiers in Cellular and Infection Microbiology 945. https://doi.org/10.3389/fcimb.2021.716789
Naga NG, El-Badan DE, Ghanem KM, Shaaban MI (2023) It is the time for quorum sensing inhibition as alternative strategy of antimicrobial therapy. Cell Communication Signal 21:133. https://doi.org/10.1186/s12964-023-01154-9
Horinouchi S (1999) γ-Butyrolactones that control secondary metabolism and cell differentiation in Streptomyces. Cell-cell Signal Bacteria 193–207
Fuqua WC, Winans SC, Greenberg EP (1994) Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. https://doi.org/10.1128/jb.176.2.269-275.1994
Tiaden A, Hilbi H (2012) α-Hydroxyketone synthesis and sensing by Legionella and Vibrio. Sensors 12:2899–2919. https://doi.org/10.3390/s120302899
Deng Y, Wu J, Tao F, Zhang L-H (2011) Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem Rev 111:160–173. https://doi.org/10.1021/cr100354f
Miller MB, Skorupski K, Lenz DH et al (2002) Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. https://doi.org/10.1016/s0092-8674(02)00829-2
Pesci EC, Pearson JP, Seed PC, Iglewski BH (1997) Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179:3127–3132. https://doi.org/10.1128/jb.179.10.3127-3132.1997
Dubern J-F, Diggle SP (2008) Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol Biosyst 4:882–888. https://doi.org/10.1039/b803796p
Chu P-L, Feng Y-M, Long Z-Q et al (2023) Novel benzothiazole derivatives as potential anti-quorum sensing agents for managing plant bacterial diseases: synthesis, antibacterial activity assessment, and SAR study. J Agric Food Chem 71:6525–6540. https://doi.org/10.1021/acs.jafc.2c07810
Tang K, Zhang X-H (2014) Quorum quenching agents: resources for antivirulence therapy. Mar Drugs 12:3245–3282. https://doi.org/10.3390/md12063245
Park J, Jagasia R, Kaufmann GF et al (2007) Infection control by antibody disruption of bacterial quorum sensing signaling. Chem Biol 14:1119–1127. https://doi.org/10.1016/j.chembiol.2007.08.013
Fetzner S (2015) Quorum quenching enzymes. J Biotechnol 201:2–14. https://doi.org/10.1016/j.jbiotec.2014.09.001
Rasmussen TB, Bjarnsholt T, Skindersoe ME et al (2005) Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J Bacteriol 187:1799–1814. https://doi.org/10.1128/JB.187.5.1799-1814.2005
Naga NG, Zaki AA, El-Badan DE et al (2022) Methoxyisoflavan derivative from Trigonella Stellata inhibited quorum sensing and virulence factors of Pseudomonas aeruginosa. World J Microbiol Biotechnol 38:1–13. https://doi.org/10.1007/s11274-022-03337-x
El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shaaban MI (2014) Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog 74:25–32. https://doi.org/10.1016/j.micpath.2014.07.008
El-Mowafy SA, Abd El Galil KH, Habib E-SE, Shaaban MI (2017) Quorum sensing inhibitory activity of sub-inhibitory concentrations of β-lactams. Afr Health Sci 17:199–207. https://doi.org/10.4314/ahs.v17i1.25
Nalca Y, Jänsch L, Bredenbruch F et al (2006) Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrobial agents and chemotherapy 50:1680–1688. https://doi.org/10.1128/AAC.50.5.1680-1688.2006.
Gabr MT, El-Gohary NS, El-Bendary ER, (2015) Synthesis, antimicrobial, antiquorum-sensing and cytotoxic activities of new series of benzothiazole derivatives. Chinese Chemical Letters 26:1522–1528
Lin Y, Xu J, Hu J et al (2003) Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum‐quenching enzymes. Mol Microbiol 47:849–860. https://doi.org/10.1046/j.1365-2958.2003.03351.x
Czajkowski R, Krzyżanowska D, Karczewska J et al (2011) Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase. Environ Microbiol Rep 3:59–68. https://doi.org/10.1111/j.1758-2229.2010.00188.x
Huang JJ, Petersen A, Whiteley M, Leadbetter JR (2006) Identification of QuiP, the product of gene PA1032, as the second acyl-homoserine lactone acylase of Pseudomonas aeruginosa PAO1. Appl Environ Microbiol 72:1190–1197. https://doi.org/10.1128/AEM.72.2.1190-1197.2006
Park S-Y, Kang H-O, Jang H-S et al (2005) Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp. and its application to quorum quenching. Appl Environ Microbiol 71:2632–2641. https://doi.org/10.1128/AEM.71.5.2632-2641.2005
Dong Y-H, Wang L-H, Xu J-L et al (2001) Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813–817. https://doi.org/10.1038/35081101
Park S-Y, Lee SJ, Oh T-K et al (2003) AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 149:1541–1550. https://doi.org/10.1099/mic.0.26269-0
Uroz S, Heinonsalo J (2008) Degradation of N-acyl homoserine lactone quorum sensing signal molecules by forest root-associated fungi. FEMS Microbiol Ecol 65:271–278. https://doi.org/10.1111/j.1574-6941.2008.00477.x
Camps J, Pujol I, Ballester F et al (2011) Paraoxonases as potential antibiofilm agents: their relationship with quorum-sensing signals in Gram-negative bacteria. Antimicrob Agents Chemother 55:1325–1331. https://doi.org/10.1128/AAC.01502-10
Faisal AJ, Said LA, Ali MR (2021) Quorum quenching effect of recombinant Paraoxonase-1 enzyme against Quorum sensing genes produced from Pseudomonas aeruginosa. Gene Rep 101412DOI. https://doi.org/10.1016/j.genrep.2021.101412
Bzdrenga J, Daudé D, Remy B et al (2017) Biotechnological applications of quorum quenching enzymes. Chemico-Biol Interact 267:104–115. https://doi.org/10.1016/j.cbi.2016.05.028
Shirazi J, Ain Q, Khan SJ et al (2021) Targeting Acyl Homoserine lactones (AHLs) by the Quorum quenching bacterial strains to Control Biofilm formation in Pseudomonas Aeruginosa. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2021.10.064
Wang N, Jian W, Liang H et al (2024) Engineering a biomimicking strategy for discovering nonivamide-based quorum-sensing inhibitors for controlling bacterial infection. Eur J Med Chem 275:116609. https://doi.org/10.1016/j.ejmech.2024.116609
Möker N, Dean CR, Tao J (2010) Pseudomonas aeruginosa increases formation of multidrug-tolerant persister cells in response to quorum-sensing signaling molecules. J Bacteriol 192:1946–1955. https://doi.org/10.1128/jb.01231-09
Maura D, Hazan R, Kitao T et al (2016) Evidence for direct control of virulence and defense gene circuits by the Pseudomonas aeruginosa quorum sensing regulator. MvfR Sci Rep 6:1–14. https://doi.org/10.1038/srep34083
Liang H, Deng X, Li X et al (2014) Molecular mechanisms of master regulator VqsM mediating quorum-sensing and antibiotic resistance in Pseudomonas aeruginosa. Nucleic Acids Res 42:10307–10320. https://doi.org/10.1093/nar/gku586
Høiby N, Ciofu O, Johansen HK et al (2011) The clinical impact of bacterial biofilms. Int J Oral Sci 3:55–65. https://doi.org/10.4248/IJOS11026
Syafiuddin A, Boopathy R, Mehmood MA (2021) Recent advances on bacterial quorum quenching as an effective strategy to control biofouling in membrane bioreactors. Bioresource Technol Rep 100745. https://doi.org/10.1016/j.biteb.2021.100745
Otto M (2006) Bacterial evasion of antimicrobial peptides by biofilm formation. Antimicrob Peptides Hum Disease 251–258. https://doi.org/10.1007/3-540-29916-5_10
Qi P, Zhang T, Yang Y et al (2024) Beyond the β-amino alcohols framework: identification of novel β‐hydroxy pyridinium salt‐decorated pterostilbene derivatives as bacterial virulence factor inhibitors. Pest Manag Sci. https://doi.org/10.1002/ps.8116
Qi P-Y, Zhang T-H, Wang N et al (2023) Natural products-based botanical bactericides discovery: novel abietic acid derivatives as anti-virulence agents for plant disease management. J Agric Food Chem 71:5463–5475. https://doi.org/10.1021/acs.jafc.2c08392
González JF, Hahn MM, Gunn JS (2018) Chronic biofilm-based infections: skewing of the immune response. Pathogens Disease 76:fty023. https://doi.org/10.1093/femspd/fty023
Michaelis C, Grohmann E (2023) Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 12:328. https://doi.org/10.3390/antibiotics12020328
Zafer MM, Mohamed GA, Ibrahim SRM et al (2024) Biofilm-mediated infections by multidrug-resistant microbes: a comprehensive exploration and forward perspectives. Arch Microbiol 206:101. https://doi.org/10.1007/s00203-023-03826-z
Zhao A, Sun J, Liu Y (2023) Understanding bacterial biofilms: from definition to treatment strategies. Front Cell Infect Microbiol 13:1137947. https://doi.org/10.3389/fcimb.2023.1137947
Gupta P, Chhibber S, Harjai K (2015) Efficacy of purified lactonase and ciprofloxacin in preventing systemic spread of Pseudomonas aeruginosa in murine burn wound model. Burns 41:153–162. https://doi.org/10.1016/j.burns.2014.06.009
Olsen I (2015) Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis 34:877–886. https://doi.org/10.1007/s10096-015-2323-z
Jakobsen TH, Bjarnsholt T, Jensen PØ et al (2013) Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors. Future Microbiol 8:901–921. https://doi.org/10.2217/fmb.13.57
Maura D, Rahme LG (2017) Pharmacological inhibition of the Pseudomonas aeruginosa MvfR quorum-sensing system interferes with biofilm formation and potentiates antibiotic-mediated biofilm disruption. Antimicrobial agents and chemotherapy 61. https://doi.org/10.1128/AAC.01362-17
Brackman G, Cos P, Maes L et al (2011) Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother 55:2655–2661. https://doi.org/10.1128/AAC.00045-11
Guo Q, Wei Y, Xia B et al (2016) Identification of a small molecule that simultaneously suppresses virulence and antibiotic resistance of Pseudomonas aeruginosa. Sci Rep 6:1–15. https://doi.org/10.1038/srep19141
Brssow H, Hendrix RW (2002) Phage genomics. Cell 108:637. https://doi.org/10.1016/s0092-8674(01)00637-7
Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. https://doi.org/10.1038/nrmicro2315
Chapman-McQuiston E, Wu XL (2008) Stochastic receptor expression allows sensitive bacteria to evade phage attack. Part I: experiments. Biophys J 94:4525–4536. https://doi.org/10.1529/biophysj.107.120212
Barrangou R, Fremaux C, Deveau H et al (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712. https://doi.org/10.1126/science.1138140
Glessner A, Smith RS, Iglewski BH, Robinson JB (1999) Roles of Pseudomonas aeruginosa Las and Rhl quorum-sensing systems in control of twitching motility. J Bacteriol 181:1623–1629. https://doi.org/10.1128/JB.181.5.1623-1629.1999
Hoque M, Mozammel IB, Naser SMN, Bari J, Zhu JJ, Mekalanos and Shah M. Faruque. 2016. Quorum Regulated Resistance of Vibrio cholerae against Environmental bacteriophages. Sci Rep 6:37956. https://doi.org/10.1038/srep37956
Tan D, Svenningsen S (2015) Lo; Middelboe, M. Quorum sensing determines the choice of antiphage defense strategy in. Vibrio anguillarum MBio 6:e00627. https://doi.org/10.1128/mBio.00627-15
Gao R, Krysciak D, Petersen K et al (2015) Genome-wide RNA sequencing analysis of quorum sensing-controlled regulons in the plant-associated Burkholderia glumae PG1 strain. Appl Environ Microbiol 81:7993–8007. https://doi.org/10.1128/AEM.01043-15
Høyland-Kroghsbo NM, Paczkowski J, Mukherjee S et al (2017) Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system. Proc Natl Acad Sci 114:131–135
Qin X, Sun Q, Yang B et al (2017) Quorum sensing influences phage infection efficiency via affecting cell population and physiological state. J Basic Microbiol 57:162–170. https://doi.org/10.1073/pnas.1617415113
Mumford R, Friman V (2017) Bacterial competition and quorum-sensing signalling shape the eco‐evolutionary outcomes of model in vitro phage therapy. Evol Appl 10:161–169. https://doi.org/10.1111/eva.12435
Hilbi H, Weber SS, Ragaz C et al (2007) Environmental predators as models for bacterial pathogenesis. Environ Microbiol 9:563–575. https://doi.org/10.1111/j.1462-2920.2007.01238.x
Clamens T, Rosay T, Crépin A et al (2017) The aliphatic amidase AmiE is involved in regulation of Pseudomonas aeruginosa virulence. Sci Rep 7:1–16. https://doi.org/10.1038/srep41178
Pukatzki S, Kessin RH, Mekalanos JJ (2002) The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium Discoideum. Proc Natl Acad Sci 99:3159–3164. https://doi.org/10.1073/pnas.052704399
Weitere M, Bergfeld T, Rice SA et al (2005) Grazing resistance of Pseudomonas aeruginosa biofilms depends on type of protective mechanism, developmental stage and protozoan feeding mode. Environ Microbiol 7:1593–1601. https://doi.org/10.1111/j.1462-2920.2005.00851.x
Ermolaeva MA, Schumacher B (2014) Insights from the worm: the C. Elegans model for innate immunity. Seminars in immunology. Elsevier, pp 303–309. DOI: https://doi.org/10.1016/j.smim.2014.04.005
Darby C, Cosma CL, Thomas JH, Manoil C (1999) Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences 96:15202–15207. https://doi.org/10.1073/pnas.96.26.15202
Lee K-M, Lim J, Nam S et al (2011) Inhibitory effects of broccoli extract on Escherichia coli O157: H7 quorum sensing and in vivo virulence. FEMS Microbiol Lett 321:67–74. https://doi.org/10.1111/j.1574-6968.2011.02311.x
Sifri CD, Mylonakis E, Singh KV et al (2002) Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect Immun 70:5647–5650. https://doi.org/10.1128/IAI.70.10.5647-5650.2002
Bijtenhoorn P, Mayerhofer H, Müller-Dieckmann J et al (2011) A novel metagenomic short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS ONE 6:e26278. https://doi.org/10.1371/journal.pone.0026278
Adonizio A, Leal SM Jr, Ausubel FM, Mathee K (2008) Attenuation of Pseudomonas aeruginosa virulence by medicinal plants in a Caenorhabditis elegans model system. J Med Microbiol 57:809–813. https://doi.org/10.1099/jmm.0.47802-0
Kong C, Eng S-A, Lim M-P, Nathan S (2016) Beyond traditional antimicrobials: A Caenorhabditis elegans model for discovery of novel anti-infectives. Frontiers in microbiology 7:1956. https://doi.org/10.3389/fmicb.2016.01956
Mahajan-Miklos S, Tan M-W, Rahme LG, Ausubel FM (1999) Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa–Caenorhabditis elegans pathogenesis model. Cell 96:47–56. https://doi.org/10.1016/s0092-8674(00)80958-7
Tan M-W, Rahme LG, Sternberg JA et al (1999) Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. Aeruginosa virulence factors. Proc Natl Acad Sci 96:2408–2413. https://doi.org/10.1073/pnas.96.5.2408
Nelson LK, D’Amours GH, Sproule-Willoughby KM et al (2009) Pseudomonas aeruginosa Las and Rhl quorum-sensing systems are important for infection and inflammation in a rat prostatitis model. Microbiology 155:2612–2619. https://doi.org/10.1099/mic.0.028464-0
Wu H, Song Z, Hentzer M et al (2004) Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J Antimicrob Chemother 53:1054–1061. https://doi.org/10.1093/jac/dkh223
Hoffmann N, Lee B, Hentzer M et al (2007) Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(–/–)mice. Antimicrob Agents Chemother 51:3677–3687. https://doi.org/10.1128/AAC.01011-06
Yin H, Deng Y, Wang H et al (2015) Tea polyphenols as an antivirulence compound disrupt quorum-sensing regulated pathogenicity of Pseudomonas aeruginosa. Sci Rep 5:1–12. https://doi.org/10.1038/SREP16158
Muhs A, Lyles JT, Parlet CP et al (2017) Virulence inhibitors from Brazilian peppertree block quorum sensing and abate dermonecrosis in skin infection models. Sci Rep 7:1–15. https://doi.org/10.1038/srep42275
Simonetti O, Cirioni O, Cacciatore I et al (2016) Efficacy of the quorum sensing inhibitor FS10 alone and in combination with tigecycline in an animal model of staphylococcal infected wound. PLoS ONE 11:e0151956. https://doi.org/10.1371/journal.pone.0151956
Balaban N, Giacometti A, Cirioni O et al (2003) Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J Infect Dis 187:625–630. https://doi.org/10.1086/345879
Abdullahi A, Amini-Nik S, Jeschke MG (2014) Animal models in burn research. Cell Mol Life Sci 71:3241–3255. https://doi.org/10.1007/s00018-014-1612-5
Saiman L, Marshall BC, Mayer-Hamblett N et al (2003) Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749–1756. https://doi.org/10.1001/jama.290.13.1749
Tateda K, Comte R, Pechere J-C et al (2001) Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 45:1930–1933. https://doi.org/10.1128/AAC.45.6.1930-1933.2001
Smyth AR, Cifelli PM, Ortori CA et al (2010) Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis—a pilot randomized controlled trial. Pediatr Pulmonol 45:356–362. https://doi.org/10.1002/ppul.21193
Walz JM, Avelar RL, Longtine KJ et al (2010) Anti-infective external coating of central venous catheters: a randomized, noninferiority trial comparing 5-fluorouracil with chlorhexidine/silver sulfadiazine in preventing catheter colonization. Crit Care Med 38:2095–2102. https://doi.org/10.1097/CCM.0b013e3181f265ba
Neoh KG, Li M, Kang E-T et al (2017) Surface modification strategies for combating catheter-related complications: recent advances and challenges. J Mater Chem B 5:2045–2067. https://doi.org/10.1039/c6tb03280j
Jain N, Bhosale P, Tale V (2016) Biofilm formation on contact lenses by bacterial pathogens. J Pharm Res 10:50–53
Francolini I, Vuotto C, Piozzi A, Donelli G (2017) Antifouling and antimicrobial biomaterials: an overview. Apmis 125:392–417. https://doi.org/10.1111/apm.12675
Mandakhalikar KD, Chua RR, Tambyah PA (2016) New technologies for prevention of catheter associated urinary tract infection. Curr Treat Options Infect Dis 8:24–41
Hraiech S, Hiblot J, Lafleur J et al (2014) Inhaled lactonase reduces Pseudomonas aeruginosa quorum sensing and mortality in rat pneumonia. PLoS ONE 9:e107125. https://doi.org/10.1371/journal.pone.0107125
Hume EBH, Baveja J, Muir B et al (2004) The control of Staphylococcus epidermidis biofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials 25:5023–5030. https://doi.org/10.1016/j.biomaterials.2004.01.048
Shenderovich J, Feldman M, Kirmayer D et al (2015) Local sustained-release delivery systems of the antibiofilm agent thiazolidinedione-8 for prevention of catheter-associated urinary tract infections. Int J Pharm 485:164–170. https://doi.org/10.1016/j.ijpharm.2015.02.067
Taunk A, Ho KKK, Iskander G et al (2016) Surface immobilization of antibacterial quorum sensing inhibitors by photochemical activation. J Biotechnol Biomater 6:1000238. https://doi.org/10.4172/2155-952X.1000238
Kim MK, Zhao A, Wang A et al (2017) Surface-attached molecules control Staphylococcus aureus quorum sensing and biofilm development. Nat Microbiol 2:1–12. https://doi.org/10.1038/nmicrobiol.2017.80
Cirioni O, Mocchegiani F, Cacciatore I et al (2013) Quorum sensing inhibitor FS3-coated vascular graft enhances daptomycin efficacy in a rat model of staphylococcal infection. Peptides 40:77–81. https://doi.org/10.1016/j.peptides.2012.12.002
Ivanova K, Fernandes MM, Mendoza E, Tzanov T (2015) Enzyme multilayer coatings inhibit Pseudomonas aeruginosa biofilm formation on urinary catheters. Appl Microbiol Biotechnol 99:4373–4385. https://doi.org/10.1007/s00253-015-6378-7
Ng FSW, Wright DM, Seah SYK (2011) Characterization of a phosphotriesterase-like lactonase from Sulfolobus solfataricus and its immobilization for disruption of quorum sensing. Appl Environ Microbiol 77:1181–1186. https://doi.org/10.1128/AEM.01642-10
Guendouze A, Plener L, Bzdrenga J et al (2017) Effect of quorum quenching lactonase in clinical isolates of Pseudomonas aeruginosa and comparison with quorum sensing inhibitors. Front Microbiol 8:227. https://doi.org/10.3389/fmicb.2017.00227
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
N. G. N. and M.M.E. wrote the main manuscript text. N.G. N. Prepared the figures. M.I.S. reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naga, N.G., Shaaban, M.I. & El-Metwally, M.M. An insight on the powerful of bacterial quorum sensing inhibition. Eur J Clin Microbiol Infect Dis (2024). https://doi.org/10.1007/s10096-024-04920-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10096-024-04920-w