Abstract
Background
Bacterial cells communicate via small extracellular molecules that facilitate gene expression which is dependent on cell density and this mechanism is known as Quorum Sensing (QS). At low cell density, these bacteria show a single cellular type of behavior but once they reach the threshold level they alter to a multicellular type and hence a QS is established by the transfer of signalling molecules called autoinducers. Quorum sensing inhibitors (QSI) are those that hinder the quorum sensing pathway.
Main body of the abstract
The emergence of antimicrobial resistance has become a threat to mankind with quorum sensing being one of the mechanisms responsible for this resistance. Hence Quorum Quenching can be considered to interrupt bacterial communication. This review focuses on the effects of different synthetic and natural quorum-sensing inhibitors on different organisms and how it affects their gene regulation.
Conclusion
Different natural and synthetic agents can quench quorum sensing by various mechanistic pathways. The various quorum-sensing inhibitors against both Gram-positive and Gram-negative bacteria provide a wider scope to prevent emerging antimicrobial resistance.
Similar content being viewed by others
Introduction
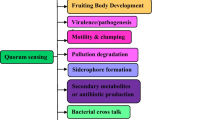
The discovery of antibiotics in the 1920s made a great revolution in the history of medicine [1]. A century later, the unrestrained, inappropriate, and continuous use of these antimicrobials led to the evolution of Multiple Drug Resistant species [2]. It was identified that one of the main reasons behind this is the biofilm formation of the bacterial colonies [3]. Other bacterial behaviors also contribute to the evolution of resistant species; this is regulated by a phenomenon called Quorum Sensing [4]. Quorum Sensing (QS) is a cell-to-cell communication between bacteria. It regulates gene expression by producing small extracellular signalling chemical molecules known as Auto-Inducers (AIs). QS is a significant mechanism for regulating various physiological activities like virulence [5], motility [6], sporogenesis [7], bioluminescence [8], and biofilm formation [9]. It also regulates other infections related to phenotypes for a signal response, usually small molecules like oligopeptides, furanones, or fatty acid derivatives [10].
Advantage that QS offers the microorganism is to control its response to environmental stimuli. The QS system also strengthens the biotransformation pathways and hence plays an important role in the biodegradation of contaminants [11].
Quorum sensing is a population-dependent mechanism, when the concentration reaches the threshold level, the signalling molecules reach a detectable level which activates the responses of the bacteria [12].
There are three mechanistic concepts through which this QS works.
-
I.
By density-dependent action—At Low Cell Density (LCD) since, there are fewer signalling molecules, it doesn’t reach the threshold level to identify the AIs. At High Cell Density (HCD) there are enough signalling molecules to establish the QS between bacterial cells, as depicted in Fig. 1.
-
II.
There are specific receptors present in the cytoplasm of a bacterial cell that identifies these signalling molecules for the QS activity to initiate.
-
III.
As the AIs get detected and activation takes place, it would therefore boost more auto-inducers [13].
The AIs are different for Gram-positive and Gram-negative bacteria [14]. In Gram-positive bacteria, the signalling molecules are known as Autoinducing Peptides (AIPs) whereas in Gram-negative bacteria the autoinducer is N-Acyl-homoserine Lactone (AHL). AI-2 are the autoinducers adopted as the communication media between the bacterial cells in both Gram-negative and Gram-positive. The secretion of these signalling molecules is by the diffusion process [15].
Quorum sensing in Gram-negative bacteria
N-Acyl Homoserine Lactone (AHL) is responsible for the signalling in Gram-negative bacteria, and it was first observed in a marine bacteria known as Vibrio fischeri. The LuxI/LuxR-type quorum sensing in Gram-negative happens through different operons. LuxI proteins are responsible for the biosynthesis of the signalling molecules specifically for the Homoserine lactone whereas LuxR binds to the autoinducers and activates the target gene transcription once they reach the threshold level (Fig. 2). On high cell density, there will be an increase in the concentration of the signalling molecules [16].
Quorum sensing for Gram-positive bacteria
The autoinducers in Gram-positive are Auto-Inducing Peptides (AIPs). The Gram-positive bacteria produce small oligopeptides in their cells. Once they mature, they are transported across their cell membrane through an ABC transporter. The AIP concentration on reaching the threshold level binds to a histidine sensor kinase segment, this then activates the kinase and successively auto-phosphorylates on histidine residue, which then passes to a response regulator protein and activates the gene expression (Fig. 3) [16].
Quorum Sensing Inhibitors
QS Inhibitors (QSI) act by inhibiting bacterial cell-to-cell communication without exerting pressure on the bacteria. Thus, QS inhibition disrupts cell-to-cell signalling and inhibits the autoinducer-induced QS system [17]. QS inhibition reduces bacterial virulence [18]. Antimicrobial resistance has been a major concern in various diseases through quorum-sensing inhibition mechanisms we can conquer this obstacle. QS inhibiting agents don’t destroy or inhibit bacterial growth, they quench QS-regulated pathogenic activities like toxin production, swimming, swarming, motility, and biofilm formation which leads to loss of the ability of bacteria to cause disease and thus drug-resistant mutations [19].
Various mechanisms of QS inhibition are as follows:
-
1.
Inhibiting the synthesis of signalling molecules [20]
-
2.
Inactivation or enzymatic degradation of signalling molecules [20, 21]
-
3.
Competing with signalling molecules [22]
-
4.
Restricting the signal receptor complex [21]
To select effective quorum-sensing inhibitors, various factors have to be considered as follows [1, 23, 24]:
-
1.
There must be a small molecule for the adequate depletion of the QS-regulated gene expression.
-
2.
There must be no adverse effect with a particular quorum sensing regulator.
-
3.
It must be chemically stable and tolerant to metabolic degradation.
-
4.
The QSI must act longer than the native AHL
There is a wide range of Quorum sensing inhibitors like:
Natural QSIs include Prokaryotic QSIs that consist of various enzymes responsible for quenching the quorum sensing activities. For example, Bacillus sp. and Agrobacterium tumefaciens consist of Lactonase enzyme that degrade the AHL signalling molecules [1, 25,26,27], another such example is Pseudomonas aeruginosa (P. aeruginosa) PAO1 consisting of the enzyme AHL-acylase that degrade the long chains of AHLs [1, 28].
Animal-based QSIs include interaction between the eukaryotic host and bacterial pathogen, which also acts by enzymatic inhibition, as seen in fungi Penicillium which consists of patulin and penicillic acid acting as a QSI by inhibiting the biofilm formation in P. aeruginosa.
Plants act as QSI by exhibiting chemicals like those of QS signals and additionally their ability to degrade the signalling receptor. In Agrobacterium tumefaciens, GABA promotes the AHL signal OHC8HSL degradation by the enzyme lactonase [27].
Synthetic QSIs outwit the limitation of lower concentration of signalling molecules in natural QSIs by chemically synthesizing them. A synthetic QSI mainly aims at the biosynthetic pathway that steers the production of signalling molecules, by changing the chain length, by substituting the signalling molecules, modifying the AHL ring moiety, and many more.
Synthetic compounds
Ibrahim et al., synthesized and evaluated 5-acetyl-4-methyl-2-(3-pyridyl) thiazole (AMPT) (Fig. 4) for the anti-quorum sensing activity against the MRSA strain. Chromobacterium violaceum produces a violet pigment called violacein through quorum sensing. Compounds that inhibit quorum sensing of the bacteria, reduce the violet colour. C. violaceum is used as a reference standard for quorum sensing inhibition sensing. It was found that AMPT can interfere with the quorum-sensing mechanism in Staphylococcus aureus and C. violaceum. The effect of AMPT on violacein of C. violaceum was observed based on the diameter of the yellowish opaque zone The plate with 4 mg/l concentration of AMPT showed the best anti-QS activity followed by 2, 1, and 0.5 mg/l concentration [29,30,31].
Rajkumari et al., evaluated 5-hydroxymethylfurfural (5-HMF) against P. aeruginosa PAO1. The QS boosts bacterial virulence by stimulating biofilm formation in P. aeruginosa. The anti-QS activity was also detected against C. violaceum using the agar well diffusion method, resulting in a non-pigmented zone of C. violaceum around a well-containing 5-HMF. 5-HMF decreased the production of C. violaceum by 98.12% at a conc. of 1.25 μL/mL. 5-HMF showed an effect against the biofilm formation of PAO1 with a MIC and sub-MIC concentration of 2.5 μL/mL and 1.25 μL/mL, respectively. 5-HMF inhibited the formation of biofilm by 29.80%. In the presence of 5-HMF, there was an inhibition of pyocyanin pigment up to 65.34%, a reduction of LasA staphylolytic activity by 77.92%, LasB elastase activity reduction by 36.48% and 57.71% reduction of chitinase activity. There was a decrease in cell surface hydrophobicity and production of eDNA by 16.01% and 17.57%, respectively. Caenorhabditi elegans infected with the PAO1 strain showed an improved mortality rate of 78.33% resulting from the effect of 5-HMF on killing C. elegans by PAO1. On treatment with sub-MIC of 5-HMF, there was a significant down-regulation by 92.79% and 90.15%, respectively, on the expression of lasI and lasR. On the exposure of 5-HMF, there was also an influence on lasB and rhlA by 59.66% and 66.20% inhibition, respectively [32,33,34].
Seleem et al., describe whether the analgesic drugs, indomethacin, and paracetamol act as quorum sensing inhibitors. In this study, P. aeruginosa O1 (PAO1) a QS positive strain and a violacein-negative mutant strain C. violaceum 026 (CV026) in the presence of AHL, the violacein purple pigment is induced. About 20 Acinetobacter baumannii isolates were used in this study. During the screening of the analgesic drugs for suppression of QS activity, it was seen that paracetamol inhibits the QS in CV026 and there was an inhibition of violacein pigment due to the halo formation around the well on purple background. Inhibition in CV026 was in presence of 1/8 MIC of paracetamol with an optical density (OD) value of 0.138 and optical density value of 0.332 in control CV026 therefore, leading to 58.4% violacein pigment inhibition. In the presence and absence of paracetamol, the virulence factors for PAO1 were determined. The MIC value of paracetamol against PAO1 was 256 μg/mL. In the presence of 1/8 MI of paracetamol, with a significant decrease in OD value (p < 0.05)from 0.723 to 0.243 at 600 nm an inhibition of biofilm formation by 66.4 ± 0.4% was found. The absorbance of culture supernatant in the absence was 0.128 and in the presence of paracetamol, it was found to be 0.085 thus showing an inhibition of pyocyanin production by 33.1 ± 0.3%. Paracetamol also inhibited the swarming motility of PAO1 by 57.1 ± 0.5%. In the presence of sub-MIC concentration of paracetamol, the twitching motility of PAO1 was inhibited by 7.7 ± 0.2%. Gelatinase production was inhibited by 17.5 ± 06% which was noted in the Gz value of 0.40 in the presence of paracetamol and the absence of paracetamol showed a Gz value of 0.33. Protease production and Rhamnolipid (in the presence of sub-MIC of paracetamol) production were inhibited in the PAO1 strain by 8.7 ± 0.4% and 33.3 ± 0.74%, respectively. Inhibition of sensitivity to hydrogen peroxide was by 9.1 ± 0.2%. The virulence factor of clinical A. baumannii isolates in the presence and absence of paracetamol were studied. Out of 20, 13, and 7 clinical isolates of A. baumannii showed the MIC of paracetamol of 512 μg/mL for MIC50 and 1024 μg/mL for MIC100, respectively. Biofilm formation was inhibited in the range of 39.7% to 93% in various isolates indicating that ≥ 50% of inhibition was observed in 75% of A. baumannii. In the presence of 1/8 MIC of paracetamol, there was an inhibition of twitching motility in the range of 6.7–82.5% with 40% isolate showing ≥ 50% inhibition of twitching motility and surface motility inhibition ranging from 7.7–29.4% with ≥ 20% inhibition of surface motility in 4 isolates. In the presence of 1/8 MIC of paracetamol, the sensitivity to hydrogen peroxide inhibition was in the range of 3.3–36.4% with 2 isolates inhibiting > 20%. The production of phospholipase in 8 isolates was detected, moderate in 2 isolates and weak in 6 isolates, the inhibition of phospholipase activity observed was in the range of 8.7–100% with 4 isolates ≥ 50% [35,36,37].
Vila-Sanjurjo et al., depicted the crosslinking of a category of nanoparticles of Chitosan with sodium tripolyphosphate (TPP) and Genipin (GNP) for its quorum quenching activity. In this study, the chitosan is first crosslinked with Genipin (as it is more compatible) and further an ionotropic formation of nanoparticles in the presence of TPP which leads to quorum quenching. The GNP Pre-Crosslinked Nanoparticles (PC-NPs) can effectively reduce the AHL-interfered quorum sensing of fluorescent biosensors in Escherichia coli. Two types of prototypes, Prototype A (PC-A) and Prototype B (PC-B) were prepared for the evaluation of the inhibition of quorum sensing activity of PC-NPs. PC-A and PC-B both showed a remarkable decrease in the fluorescence response of biosensor FI/OD600 but while comparing the two prototypes PC-B was more active than PC-A, with a decrease in the endpoint FI/OD600 in the range ~ 50–92% and ~ 35–85%, respectively. Along with the fluorescence reduction, their growth inhibition was also significantly minimized but the growth impairment magnitude by the PC-NPs was lesser than the fluorescence. The study also provided the data of standard deviation and mean in the experiment with replicates providing a p value of p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001 [38,39,40].
Molnar et al., have come up with an ingenious approach for Cyclodextrin (CD) mediated inhibition of quorum sensing. The bioluminescence of A. fischeri was investigated. Various native cyclodextrin was considered in this article such as α-, ß- and γ-cyclodextrins (ACD),(BCD) and (GCD), 2-hydroxypropyl (HPACD, HPBCD) trimethylaminopropyl (QAACD), random methylated (RAMEA, RAMEB, RAMEG), sulfobutyl ether (SBEBCD) derivatives and their epichlorohydrin-crosslinked polymer (ACDPS and BCDPS). There was a significant reduction in the bioluminescence with the highest inhibition observed was ~ 64% after 120 min obtained by 10 mM ACD; it was also low as 0.625 mM ACD concentration after 120 min. Inhibition of enzyme activity by the addition of ACD by 24% for 5 mM and 29% for 10 mM. BCD correspondingly to ACD showed an inhibitory effect with an increase in time and concentration but comparatively, the effect of BCD was less than ACD. Out of the studied BCDs, the SBECD revealed high QS inhibition ~ 30% at 5 mM concentration at 120 min. RAMEB and BCD exhibited a minute stimulatory effect < 20% on the bioluminescence at 30 min and 60 min at low concentration, whereas no effects showed at 120 min. With this, it was concluded that there were no significant antibacterial effects offered by BCD.
The data obtained Table 1 summarizes the efficiency order of cyclodextrins are: BCDPS < ACDPS, SBEBCD < RAMEB < QAACD < HPACD < BCD < RAMEA < ACD.
Efficiency order of cyclodextrin based on the MIC given in Table 1 are as follows: ACDPS, QAACD, RAMEB < ACD, BCDPS, HPACD, RAMEA, SBEBCD < HPBCD, BCD.
The low MIC of BCD and HPBCD indicates it can be considered for its effective quorum quenching activity. Based on the correlation coefficient(r) the ACD showed a well-built correlation between ACD concentration and bioluminescence at r > 0.85 at both time intervals depicting the great QQ effect of ACD [41,42,43].
Liu et al., had evaluated the quorum-quenching effects of aromatic furanones and brominated pyrrolones. They additionally designed and synthesized a new series of aryl-substituted pyrrolones. Based on biofilm formation and pyocyanin production, the QS inhibition was evaluated. In this study, there is a structural optimization of brominated furanone and pyrrolidones. First, the aromatic furanones were designed and synthesized, and then the aryl-substituted pyrrolidone derivatives were designed and synthesized for their QQ activity based on the series of furanones and pyrrolidones. The aromatic furanone didn’t show antibacterial activity in the case of both Gram-positive and Gram-negative bacteria, they also had no effect against the metabolic activity, whereas a slight effect on its growth. In comparison with it, the brominated pyrrolidone derivatives showed a fair inhibitory effect on Gram-positive bacteria including S. aureus ATCC25923 and S. aureus ATCC43300, and Bacillus subtilis ATCC9372. It didn’t show any effect against Gram-negative bacteria including E. coli ATCC25922 and P. aeruginosa PAO1. Since the bacterial cell wall of Gram-negative bacteria has a protective action against the antibacterial agents, the pyrrolidone derivatives exhibited a little inhibitory effect against Gram-negative bacteria such as E. coli and P. aeruginosa (Table 2).
Inhibition of pyocyanin production
Table 3 provides inhibition of pyocyanin production and protease activity of Compound 1 and Compound 3. Out of 6 compounds that exhibited potent inhibition about 80% of pyocyanin production with an OD520 value of 0.0082. Compounds 2, 3, and, 4 were inhibited by 43.3%, 52.6% and 43.1%, respectively. All six compounds showed protease inhibitory effect, in which compound 1 exhibited as the most potent inhibitor with 78.5% followed by compound 4 with 75.6%, the remaining 4 compounds were in ratios around 65%. As the inhibitory effect of compound 1 (Fig. 5) and 3 (Fig. 6) was more than 50% their IC50 values were also determined.
Based on the dose–response curve, it was observed that at high concentrations there was an increase in the inhibition rate of protease activity while at low concentrations there was an increase in the inhibition of pyocyanin production. Compound 1 showed better inhibition of biofilm formation with IC50 value 0.260 ± 0.035 mM and the coefficient of determination i.e., R2 of 0.885 whereas compound 4 didn’t exhibit any inhibitory effect against biofilm formation [44,45,46].
Bueloni et al., designed a complex of wide spectrum antibiotics that is nalidixic acid (NA) and oxidovanadium (IV) ion, and its incorporation into hybrid nanoparticles were also inspected. In this study, an encapsulation of vanadium and nalidixic acid (V-NA) is done. The swarming ability of Gram-positive bacteria and the quorum sensing of Gram-negative bacteria are evaluated. Chi-coated and Eudragit nanoparticles and myristyl myristate NLCs were suggested as good bio carriers for encapsulation. The complex V-NA showed better antibacterial results than the NA ligand with twofold decrease in the MIC and IC50 values (Table 4). There was a great inhibition observed in E. coli and B. cereus in both strains whereas lesser antibacterial effect concerning biofilm formation in P. aeruginosa and S. aureus.
An antibiogram assay was also done, which provided a more enhanced result compared to the MIC values. At the conc. of 10 mM an increase in the inhibition of halo formation was detected against P. aeruginosa and S. aureus. A halo increase ranging from 46 to 65% was observed for V-NA in contrast with NA indicating the effectiveness of the antibacterial activity with complexation. When treated with sub-inhibitory conc. of V-NA there was a significant reduction in swarming motility seen in S. aureus and P. aeruginosa, 2.5 to 10 times lesser compared to CIM value at 1,2, and 7 days. Quorum quenching when V-NA was encapsulated with NLCs and EuNPs, was evaluated based on the production of violacein pigment by C. violaceum. From the obtained results, it showed that the encapsulated formulation with nanoparticles showed better loss of pigment of C. violaceum whereas the free V-NA didn’t exhibit a loss of pigment [47,48,49].
Chang et al., designed, synthesized various derivatives of halogenated furanones with 5-hydroxyl-3,4-halogenated-5H-furan-2-ones as a precursor and substituting with alkyl chains, vinyl bromide or aromatic rings. An evaluation of its biofilm formation in P. aeruginosa was done on the synthesized compounds. Compound 5 (Fig. 7) and 6 (Fig. 8) showed a MIC value of 32 μg/mL for strain ATCC9027.
There was a remarkable inhibition of pyocyanin production of P. aeruginosa when treated with phenolic compounds and their derivatives. Compound 7 ((Fig. 9) exhibited the best results in the depletion of pyocyanin production along with the inhibition of biofilm formation. There was a reduction in the GFP fluorescence that indicates the presence of 3-oxo-C12-HSL antagonist and thereby inhibition of lasB gene. With GFP values with OD600, it was proved that compound 7 was effective in a dose-dependent manner for the inhibition of lasB expression [50,51,52].
Jiang et al., have designed and synthesized eighteen oxazolidinone derivatives with 3-amino-2-oxazolidinone compounds using ZS-12 as the lead compound. The compounds synthesized were evaluated against the quorum quenching activities on the CV026 strain of C. violaceum. An evaluation was done on the synthesized compounds there was an inhibition of violacein production observed in CV026 by considering C10-HSL as a positive control. Compound 8 (Fig. 10) showed the maximum inhibitory effect in C. violaceum with IC50 1⁄4 3.686 ± 0.5790 mM. The inhibition of biofilm formation in P. aeruginosa was found in the range of 42.98%—17.67%. The inhibition was concentration-dependent, for the compound 8 at 162.5 mM (p < 0.05) was 40.39%. With 162.5 mM as the concentration of compound 8, the suppression observed was 22.53% (p < 0.01) for pyocyanin, 16.25% (p < 0.01) for elastase, 57.91% (p < 0.01) for rhamnolipid and 14.91% (p < 0.01) for protease. There was also a decrease in the swarming motility when treated with the compound concerning the control [53,54,55].
Marjani et al., has detected the inhibition of biofilm formation in A. baumannii using 1,3-oxazole and imidazole-5-one. The evaluation of biofilm formation was done on 127 A. baumannii out of which 119 that is 93.7% were biofilm producers. 5 isolates were taken and the test and control isolates were evaluated. The difference between the rate of biofilm formation of test and control isolates was estimated at a significant level of p > 0.01. From the results obtained the lowest biofilm formation was observed in compound 9 with a value of 0.06350 ± 0.003536 and compound 10 with a value of 0.06350 ± 0.000707 against A. baumannii 114 and it was evaluated to the rate of control with a value 0.143 [56,57,58].
Yang et al., have synthesized 22 quorum quenching derivatives of 3-Hydroxy-2,3-dihydroquinazolin-4(1H)-one that mimics the structure autoinducer and acinetobactin. To understand the effect of inhibition of biofilm formation 3-Hydroxy-2,3-dihydroquinazolin-4(1H)-one was modified at positions R and R1 (Table 5).
Biofilm inhibition was evaluated using crystal violet stain and at four different concentrations of 500 μM, 250 μM, 50 μM and 10 μM. Inhibition of biofilm formation of compound 11 and Compound 12 was evaluated against A. baumannii, E. coli, and P. aeruginosa considering (S)-2-(3-bromophenyl)-N-(2-oxotetrahydrofran-3-yl)acetamide(3-BrBAHSL) as the positive control (Table 6). Among the derivatives obtained, compound 11 and 12 (Fig. 11) showed significant suppression of biofilm formation by 31% and 34%, respectively, proving to be the best quorum-quenching inhibitors. These two compounds (11 and 12) were then evaluated against Gentamycin sulfate. The MIC value, FIC (Fractional Inhibitory Concentration), and FICI value were established, which showed a synergistic effect (Table 7).
Hence 2-hydroxyphenyl at 2nd position is critical for inhibition of biofilm formation. And there will be a synergistic effect with Gentamycin [56, 59, 60].
El-Khouly et al., designed and synthesized various benzofuran-based derivatives. The quorum quenching activity of the Compounds 13, 15, 16, 17, 18, 19, 20, and 21 (Fig. 12) against both Gram-positive and Gram-negative bacteria with ampicillin as the reference drug was evaluated (Table 8). In the Gram-positive bacteria, the compounds 15, 16, 17, 18, 19 and 20 with respect to ampicillin exhibited a good inhibition zone in S. aureus, likewise compound 13, 15, 16, 17, 18 and 19 showed inhibition zone with respect to ampicillin in Bacillus cereus. In Gram-negative bacteria, compounds 17, 19, 20 and 21 showed the inhibition zone in P. aeruginosa. The quorum sensing inhibitions were evaluated based on the violacein production of C. violaceum and from the compounds synthesized, compound 13 and 14 exhibited quorum quenching effect.
Compound 13: (E)-2-(2-(1-(benzofuran-2-yl)ethylidene)hydrazineyl)-4-methylthiazole, Compound 15: (E)-3-(benzofuran-2-yl)-3-hydroxy-2-((E)-(4-methoxyphenyl) diazenyl)acrylaldehyde, Compound 16: (E)-3-(benzofuran-2-yl)-3-hydroxy-2-((E)-(4-cholorophenyl) diazenyl)acrylaldehyde, Compound 17: (E)-3-(benzofuran-2-yl)-3-hydroxy-2-((E)-(4-methylphenyl) diazenyl)acrylaldehyde, Compound 18: (E)-3-(benzofuran-2-yl)-3-hydroxy-2-((E)-(3-methylphenyl) diazenyl)acrylaldehyde, Compound 19: (E)-3-(benzofuran-2-yl)-3-hydroxy-2-((E)-(2-methylphenyl) diazenyl)acrylaldehyde, Compound 20: (E)-3-(benzofuran-2-yl)-3-hydroxy-2-((E)-(4-nitrolphenyl) diazenyl)acrylaldehyde, and Compound 21: 2-(4-methoxyphenyl)-2-oxoethyl (E)-2-(1-(benzofuran-2-yl)ethylidene)hydrazine-1-carbodithioate
The MIC of compounds synthesized were evaluated in comparison with ampicillin (Table 9). The compounds 19, m-Cl substituted, 18, p-NO3 substituted showed the best inhibition against S. aureus with a MIC value of 8 μg/mL [61,62,63].
Nagasundaram et al., synthesized a series of azo fused 2,3-dihydro-1H-perimidine scaffolds and its antibacterial activity were evaluated further the compound that showed the best results were evaluated for its various bacterial functions that is governed by the quorum sensing activity like biofilm formation, swimming and swarming motility activity. The quorum quenching activity was evaluated against C. violaceum. The synthesized compound was (aryldiazenyl) − 2-(2,3-dihydro-1H-perimidin-2-yl) phenol and its derivatives. The antibacterial activity of the derived compound was evaluated based on the inhibition of the zone (Table 10), tested against various Gram-positive and Gram-negative bacteria and was examined using 50 μL.
Based on the antibacterial activity, it was observed that the Compound 22 and 23 (Fig. 13) showed the better activity among others against P. aeruginosa. Hence the two azo fused compounds were tested for antibiofilm formation. From the evaluation of biofilm formation against P. aeruginosa, the result obtained were showing a significant reduction in the biofilm production with MIC value 1.56 μg/mL for compound 22 and 3.13 μg/mL for compound 23 and the percentage obtained gives a comparison between 22 and 23, 22 is a p-nitro substituted compound with 37.08 and 23 is a m-nitro compound with 30.23% and Rifamycin that is taken as the standard positive control shows 52.75% of inhibiting biofilm formation (Table 11).
The compounds 22 and 23 showed a significant decrease in the swimming and swarming motility of P. aeruginosa. The quorum quenching was also evaluated against C. violaceum CV026, the compound 22 and 23 at a concentration of 50 μL, showed a remarkable reduction in the violacein production with Rifamycin taken as a control (Table 12).
From the results obtained (Table 12), it is evident that 22 offers a stronger quorum quenching, anti-swimming, and anti-swarming activity than 23 [64,65,66].
Hopa et al., has synthesized novel mixed-ligand two Co (II) complexes, Compound 24-[Co(btmpp)(NCO)2] and Compound 25-[Co(btmpp)(NCSe)2] in which btmpp is 2,6-bis(3,4,5-trimethylpyrazolyl)pyridine). These complexes were tested against both Gram-positive and Gram-negative bacteria. The quorum sensing behavior in bacteria like swarming motility and biofilm production were evaluated. The MIC values expressed in mM, were evaluated by comparing the compound 24 and 25 with the standard drug (Table 13). From the values obtained compound 25 showed better results against P. aeruginosa and S. sonnei whereas compound 24 showed better results when it was evaluated against Yersinia enterocolitica in comparison with azithromycin and ampicillin which was taken as the standard drug. Antibacterial activity was evaluated based on the MIC values against various bacterial strains for both compound 24: [Co(btmpp)(NCO)2] and compound 25: [Co(btmpp)(NCSe)2]. The bacterial strains were more sensitive to compound 25 than for 24, also the inhibition of compound 25 was evaluated in contrast to S. sonnei with 30 mm and compound 25 was evaluated in contrast to P. aeruginosa with 21 mm. Hence compound 25 exhibited better antibacterial activity than compound 24 and this might be due to the selenocyanate group present in compound 25 (Table 14).
There was a complete inhibition of the bacterial strain with the treatment of compound 25 whereas there was an increase in the activity against swarming of bacterial strains when treated with compound 24. As the rhl system is the key contributor to the swarming ability in P. aeruginosa, the results obtained indicate that the rhl inhibitor is present in compound 25 (Table 15).
When the activity of the complex against the biofilm formation was evaluated compound 25 showed significant results in the range of 1–0.001 mM in contrast to it, compound 24 in the range of 10–0.01 mM, didn’t show significant results against S. sonnei and P. aeruginosa [67,68,69].
Qin et al., synthesized and characterized compounds that are structurally related to homoserine γ-lactone, these compounds are known as TGK-series and its quorum quenching activity was evaluated against E. coli. Hence in this study, the TGK series of compounds hinder the potential of the bacteria to produce GFP at 50 μM concentration. The compound TGK series were evaluated against E. coli top 10 strains for its potential to interfere with the GFP fluorescence produced by 3OC6HSL. The compounds TGK2, TGK3, and TGK4 suppressed the quorum sensing activity by 53.5% (p ≤ 0.01), 56.9% (p ≤ 0.01) and 57.9% (p ≤ 0.01), respectively, as these compounds didn’t have any substituents added with it whereas TGK1 compound had two benzene rings attached with methyl group at the para position with respect to methylene connected to nitrogen in that molecule hence there was a stronger quorum sensing inhibition by 47.9 (p ≤ 0.001) observed in compound TGK1 [70,71,72].
Maruthupandy et al., had prepared Nickel oxide nanoparticles (NiO NPs) and its quorum quenching and antibiofilm activity were evaluated. The violacein inhibition of C. violaceum was also evaluated. The antibacterial activity was evaluated at 50 μg/mL to check the zone of inhibition. Ceftazidime which was taken as the positive control exhibited a zone of inhibition of 6 mm. The Nickel oxide nanoparticles exhibited a zone of inhibition of 7 mm at 10 μg/mL concentration and 12 mm at 5 μg/mL concentration hence indicating that the antibacterial effect of the NiO NPs is concentration-dependent and with an increase in concentration the antibacterial effect also increases. The introduction of NiO NPs with C6-HSL to C. violaceum CV026 showed an excellent inhibition of violacein up to 90% at a concentration of 60 μg/mL. The NiO NPs at concentration of 60 μg/mL showed 94% of inhibition of biofilm formation against P. aeruginosa with respect to the control which indicates activity against biofilm formation also increases with increase in the concentration of NiO NPs [73,74,75].
Li et al., describes the effect of Methyl anthranilate at sub-MIC30 for evaluating the phenotypes that are regulated by quorum sensing against Aeromonas sobria. At a concentration ranging from 0.125μL/mL to 5 μL/mL the MIC value of methyl anthranilate was determined. The MIC value of C. violaceum CV026 was 2.0 μL/mL and A. sobria was 5.0 μL/mL. The inhibitory effect of methyl anthranilate to produce violacein was determined on C. violaceum CV026, it was observed that there was concentration-dependent inhibition of violacein. At a concentration of 0.5 μL/mL of methyl anthranilate, the maximum inhibition rate of violacein production was 41.01% with no growth inhibition. The determination of the effect of methyl anthranilate on the biofilm formation was done by treating methyl anthranilate with different sub-MICs against A. sobria. A remarkable suppression of biofilm formation was observed to the level of 31.67%, 34.01%, 41.27% and 51.44% when treated with 0.5μL/mL, 0.25 μL/mL, 0.125 μL/mL and 0.0625 μL/mL values of sub-MIC, respectively, against A. sobria. The swimming and swarming motility of A. sobria at a concentration of 0.5μL/mL of methyl anthranilate showed a gradual reduction with the maximum inhibitory rate of 74.84% and 71.63%, respectively. The protease activity of A. sobria was also evaluated. At a concentration of 0.5 μL/mL of methyl anthranilate there was a reduction of up to 43.08% of A. sobria. The incorporation of C4-HSL the protease activity enhances. With the treatment of 0.5 μL/mL methyl anthranilate the minimal concentration of C4-HSL was reduced to 0.865 μL/mL [76,77,78].
Salini et al., evaluates the biofilm formation of Vibrio harveyi when treated with undecanoic acid (UDA), the auxins such as Indole-3-Acetic Acid (IAA) and Indole-3-butyric acid (IBA) individually, and in combination. The MIC values of UDA was 20 μg/mL and IAA or IBB was 200 μg/mL, respectively (Table 16). The biofilm formation of V. harveyi was evaluated based on FICI value which was found to be 0.65 when UDA and auxins were combined (Table 16).
The biofilm formation was evaluated both individually and in combination. With a maximum conc. of 20 μg/mL the compounds undecanoic acid, Indole-3-Acetic Acid, and Indole-3-butyric acid, suppressed the biofilm formation up to 18.9%, 14.5%, and 8.6%, respectively, whereas on combination the reduction of biofilm formation was concentration dependent of UDA with IAA or IBA in the range of 21–79.7% and 25.8–60.1%, respectively. It was also observed that there was a concentration-dependent reduction during the evaluation of bioluminescence on V. harveyi. The reduction in bioluminescence with the combination of the UDA (at a conc. of 10 μg/mL) with IAA or IBA (at a conc. of 10, 20 and 30 μg/mL) observed were at a range of 75.9–93.6 and 13.2–76.7%, respectively [79,80,81].
Saqr et al., describes Allopurinol as a quorum quenching agent when evaluated for the various virulence factors that are regulated by the QS system. The determination of MIC values of Allopurinol against P. aeruginosa was done and the least concentration that hindered the growth of P. aeruginosa PAO1 was 2 mg/mL. There was a reduction of violacein production by 60% observed on C. violaceum CV026 when treated with allopurinol at 1/10 MIC. There was an inhibition of biofilm formation by Allopurinol against P. aeruginosa PAO1 by 61%. On the treatment with Allopurinol at sub-MIC, the ability of P. aeruginosa to swim was reduced to 92%, the twitching ability was reduced to 87%, and swarming motility was reduced to 85%. Other virulence factors such as hemolytic activity, elastolytic activity, pyocyanin production, and rhamnolipid production (which is determined by clearance zone) was significantly reduced by Allopurinol against P. aeruginosa by 95%, 93%, 74% and 74%, respectively. The expression of QS genes was also tested and showed a decrease in the expression of P. aeruginosa genes by Allopurinol at sub-MIC [82,83,84].
Natural compounds
Zhu et al., explored the effect of exudates taken from the root of Sedum alfredii regulated by quorum sensing on the bacteria P. aeruginosa. The quorum sensing ability of S. alfredii root extracts were evaluated against P. aeruginosa PAO1(WT), green fluorescence reporter (gfp-lasI), and the rhl QS mutant strain (ΔrhlI). When the root extracts were evaluated for its quorum quenching activity, it was observed that the protease synthesis of WT and ΔrhlI were inhibited by 51.4% and 30.4%, respectively, and the protease concentration of WT was reduced to 0.36 mg/L and of ΔrhlI was reduced to 0.32 mg/L. The screening of quorum sensing inhibition was analyzed, and several substances were found like leucine, serine, threonine, aspartic acid, glycerol, sorbitol,2-piperidine carboxylic acid, squalene, tropone, methyl oleate, monolinolein and thymol. Out of these substances obtained thymol showed the best inhibition among other substances. At a concentration of 50 μmol/L of thymol there was a reduction of protease activation by 44.7% and 33.7% in WT and ΔrhlI, respectively, in comparison with the control and rate of inhibition approximately 24.3% when evaluated on P. aeruginosa. The fluorescence expression of gfp-lasI represents the gene expression of 3-oxo-C12. The resulting gene avg. gfp-las expressions obtained were 1.64 × 106, 1.71 × 106, and 1.72 × 106/OD600 for untreated, 1 × root exudates, and for thymol at a concentration of 50 μmol/L. It was also observed that there is an inhibition of expression of lasR gene and transcription of elastase by treating it with thymol on P. aeruginosa. In order to understand the transcriptional expression of lasR and lasB genes a qRT-PCR test was done and lasB gene showed significant reduction in the gene expression rate by 36.0% for 1 × root exudates and 73.0% for 50 μmol/L thymol, but no result was found by lasB gene [85,86,87].
Ya Fei Geng et al., describes luteolin the ability to inhibit quorum sensing when evaluated against P. aeruginosa. The Minimum Inhibitory Concentration (MIC) values obtained was 1 mM for luteolin whereas the value obtained for positive control (quercetin) was greater than 1 mM. At sub-MICs 50, 100 and 200 μM, 100 μM luteolin had the maximum inhibition of biofilm formation (p < 0.01). At the sub-MICs 50 μM, 100 μM and 200 μM, 200 μM luteolin showed a remarkable reduction in pyocyanin production (p < 0.01). The elastase activity was significantly reduced by luteolin. Likewise, it was observed that there was a great decrease in the rhamnolipid of 100 μM luteolin that showed a similar reduction effect as that of quercetin 100 μM. The swimming ability was significantly suppressed with 200 μM luteolin showing the maximum inhibition (p < 0.01) and swarming motility showed intermediate inhibition at 200 μM, in comparison with the positive control (p < 0.05) [88,89,90].
Shukla et al., describes gingerol as an effective quorum sensing inhibitor when evaluated against P. aeruginosa. At 30 μg/mL of gingerol, there was a decrease by 30%, 40%, 40%, and 20% for biofilm formation, Extracellular polymeric substance (EPS) production, pyocyanin production and rhamnolipid production, respectively. The reduction in LasR led to the suppression of biofilm formation and EPS. PhzR and RhlR is controlled by LasR and the suppression of PhzR has an effect on pyocyanin and RhlR on rhamnolipid. The potency of ciprofloxacin, an antibiotic, was increased with gingerol by 20% with a minimal conc. of 0.5 μg/mL and at 1.0 μg/mL of ciprofloxacin the effect was doubled [91,92,93].
Abdulrahman et al., used curcumin-mediated antimicrobial photodynamic therapy (APDT) for quorum quenching in P. aeruginosa. APDT is a type of treatment that involves a combination of visible light and photosensitizer(PS) in presence of oxygen. Cell death occurs when this photosensitizer absorbs light of a particular wavelength that leads to the formation of reactive oxygen species. Curcumin was evaluated after the APDT and there was an inhibition in the biofilm formation by 23% without light, 40% and 70% inhibition by 5 J/cm2 and 10 J/cm2 light, respectively. With laser light 10 J/cm2 showed more effectiveness than 5 J/cm2 and curcumin alone. It was observed that there was a major downregulation of genes with 10 J/cm2 light doses [94,95,96].
Prateeksha et al., presented isolated ELF from lichen and Usnea longissima Ach to determine its effect on quorum sensing inhibition. The fungal extract known as metabolites extract (MELF) was evaluated for its quorum quenching activity. The decrease in the violacein production in C. violaceum ATCC 12472 was evaluated and it showed a concentration dependent inhibition. At 6 mg/ml of MELF violacein inhibition was by ~ 81% whereas naringenin which was taken as a positive control was inhibited by ~ 72% at 0.5 mg/ml concentration of violacein production. There was an inhibition of pyocyanin production by ~ 75% at 6 mg ml concentration whereas naringenin showed 70% at 0.5 mg ml concentration. At 6 mg/ml of MELF showed 77% proteolytic inhibition wherein the positive control showed 79% at 0.5 mg/ml. There was 16%, 42% and 69% reduction of elastolytic activity at 2, 4 and 6 mg/ml of MELF and the positive control showed inhibition of ~ 61% in elastase activity. MELF at 6 mg/ml showed 79% and 72% decrease in rhamnolipid and extracellular polysaccharides, respectively [97,98,99].
Chang et al., describes the quorum quenching ability of chrysin which was isolated from Penicillium chrysogenum DXY-1. Tyrosol is also an active molecule obtained from the extract which was evaluated along with chrysin. The inhibition of violacein production in C. violaceum was found to be concentration dependent. At a concentration of 20 μg/mL chrysin inhibited violacein production by 31.6% in comparison with the negative control i.e., DMSO, whereas the inhibition of violacein production of DXY-1 metabolite at 20 μg/mL was found to be 21.5% and 17% violacein inhibition by tyrosol at a very high concentration of 100 μg/mL. 41.4% of pyocyanin inhibition of 40 μg/mL of chrysin was obtained, 13.8% of suppression in the elastase activity and 8.3% of proteolytic activity when compared to the negative control. At 100 μg/mL of tyrosol only 8.5% of inhibition of pyocyanin production was observed. The positive control AZM with a concentration of 50 μg/mL dose, 77.0%, 79.1% and 75.9% inhibition of pyocyanin production, elastase activity and proteolytic activity, respectively, was observed. At 40 μg/mL concentration of chrysin there was a suppression of 42.4% of biofilm formation whereas AZM which is taken as a positive control inhibited by 76.7%. The resulting value shows that there is concentration dependent decrease overall [100,101,102].
Ghoreishi et al., have investigated the effectiveness of the extracts from Halobacillus karajensis of their pyocyanin and biofilm formation inhibition against S. aureus and P. aeruginosa. From the results obtained the crude supernatant extract, the middle phase of Methanol/Chloroform Supernatant extract (MMS) from H. karajensis showed the best reduction in biofilm formation in S. aureus by 74%, and P. aeruginosa by 27%. The protein profile of MMS was evaluated for its pyocyanin inhibition and the most effective protein detected was the fragment with the lightest molecular weight of 25 kD with 60% of pyocyanin reduction [103,104,105].
Molina et al., have evaluated different Laurus nobilis extracts based on their polarity such as n-hexane (HE), Chloroform (CE), ethyl acetate (EAE), methanol (ME) and total methanol extract (TME). At a concentration of 100 μg/mL the Escherichia coli strains showed an inhibition of biofilm formation below 40%. S. aureus strains inhibited biofilm formation up to 76% and 55% by the strains ATCC 6538 and ATCC 25904, respectively. The only extract capable of inhibiting biofilm formation against all the strains was HE at a concentration of 100 μg/mL. A significant inhibition was also observed in CE and EAE extracts against E. coli, P. aeruginosa and S. aureus. HE and CE were evaluated if it could protect the surface against biofilm formation hence it was covered with 10 μg/mL and 100 μg/mL of polystyrene fragments. There was a decrease detected in biofilm biomass and activity between 40 and 60%. The swimming motility was determined against P. aeruginosa PAO1 against the extracts EAE, HE, CE and TME all were above 30%. At 100 μg/mL, there was a decrease in pyocyanin by 23%, 35%, 50% and 54% for HE, TME, CE and EAE, respectively, and 43% and 45% reduction in the elastase activity by 29%, 43% and 45% for TME, HE and CE, respectively [106,107,108].
Ahmed et al., investigated the inhibition of virulence factor of P. aeruginosa by isolated plant compounds such as trans-cinnamaldehyde (CA) and salicylic acid (SA). The decrease in biofilm formation was better detected in SA than CA. The reduction in biofilm formation was 54% and 26% of SA and CA, respectively, but when CA and SA were combined, they showed an efficient reduction of 62%. The lasA protease activities of SA and CA were evaluated, the OD440 dropped from 0.3 to 0.1 for 31% reduction (p < 0.05) in absorbance reading in the presence of SA whereas in the presence of CA a better reduction of 65% (p < 0.01) was observed. The combined effect of CA and SA showed the best reduction of up to 80% (p < 0.001) in comparison with the untreated PAO1. LasB elastase activity in presence of CA showed a decrease of OD495 from 0.08 to 0.06 with reduction of 22% (p < 0.01) whereas SA showed OD495 reduction with 28% and when combined exhibited better reduction of up to 46%. The pyocyanin production in presence of CA and SA were decreased from 3.1 μg/ml to 2.1 μg/ml and 0.922 μg/ml, respectively, and in combination reduced up to 1.1 μg/ml with an yield of 64%. The decrease in the yield of rhamnolipid in the presence of CA and SA was approx. from 1.72 g/l and 0.7 g/l [109,110,111].
Mu et al., investigated the quorum sensing inhibitory activity on violacein production of Coreopsis tinctoria Nutt. When the concentration of the crude extract of Coreopsis tinctoria Nutt is ≥ 0.25 mg/mL there was a reduction of violet production in C. violaceum ATCC12472 by ≥ 50% and at 4 mg/mL it decreases up to ≥ 90%. The violacein production was also detected by using different fractions and hence 50% and 95% MeOH fractions of the crude extract possessing the highest inhibition on violacein production. These were separated and identified and out of which the Okanin separated from 95% MeOH played an important role in inhibiting the violacein production. There was an inverse correlation between okanin concentration and the intensity of purple pigment, at a concentration of 7.81 μg/mL of okanin was found to decrease significantly (p < 0.05) in comparison with untreated control whereas with at least 15.63 μg/mL of okanin developed turbid yellowish suspensions with no hint of purple depicting the bacterial growth in absence if violacein production. The effect of violacein production by Okanin via influencing the expression of vioABCDE operon, founded that at a concentration of 62.5 μg/mL of C. violaceum ATCC12472 showed a downregulation of vioD and vioE by two-fold and vioB by four-fold in comparison with the untreated control. The best result was obtained by vioA by at least 15-fold of Okanin treatment [112,113,114].
Wei et al., investigated the effect of Phloretin an antibacterial on Listeria monocytogenes biofilm. At a concentration of 20 μg/mL of Phloretin, L. monocytogenes showed an inhibition of biofilm production up to 60% at the maturation stage and there was a decrease in the biofilm thickness by 2 μm approximately. At the same concentration, there was a decrease by 50% in the transcription level of the agrosystem [115,116,117].
Guzman et al., explored the effect of Piper betle L. leaf extract such as rude ethanolic extract (CE) and crude alkaloids (CA) were investigated for the ability to inhibit the quorum sensing activity of Shrimp pathogen V. harveyi. The inhibition of biofilm formation without inhibiting their growth was found to be in a concentration dependent manner, at 100 μg/mL concentration CE (p < 0.05) inhibited biofilm formation on V. harveyi VH0, V. harveyi VH1 and V. harveyi BAA-1116 by 58.88%, 56.42% and 57.89%, respectively. At 50 μg/mL concentration of CA (p < 0.05), the inhibition of biofilm formation on. harveyi VH0, V. harveyi VH1 and V. harveyi were by 6.77%, 54.41% and 55.05%, respectively. At 100 μg/mL concentration of CE (p < 0.05) the bioluminescence of in V. harveyi BAA-1116 strain was reduced up to 64% and significant reduction in lower concentrations. Considering the CA at 50 μg/mL, 25 μg/mL, and 12 μg/mL the bioluminescence inhibition (p < 0.05) was found to be 50%, 30%, and 10%, respectively. Piper betle CE and CA also inhibited the QS via AI pathways in a concentration dependent manner. At 100 μg/mL concentration of CE AI-2 affected by 97% whereas AI-1 by 83%, similarly 88% by AI-1 and 87% by AI-2 was observed with 50 μg/mL concentration of CA [118,119,120].
Wang et al., investigated the inhibition of quorum sensing activity of Pseudomonas fluorescens and Shewanella baltica in seafood products and the main QS regulators such as LuxR/I family were evaluated. The MIC of 5′-CMP and 5′-AMP inhibiting P. fluorescens PF08 were 0.155 mmol/L and 0.3 mmol/L, respectively, and those inhibiting S. baltica OS155 were 0.139 mmol/L and 0.130 mmol/L. 5’-CMP and 5-AMP could inhibit AHL and DKP production in P. fluorescens PF08 and inhibition of DKP production in S. baltica OS155. C4-HSL is an QS signalling molecule that was reduced by 58.4% and 55% when P. fluorescens PF08 was treated with 5′-CMP and 5′-AMP [121,122,123].
Danaraj et al., have reported the QS inhibitory activity of the active constituents present in the seagrass Halodule pinifolia against P. aeruginosa PAO1. The leaves were extracted using chloroform and methanol and the biofilm formation was obtained maximum by the methanolic extract at 35 μg/mL in comparison with Cephalosporin at 25 μg/mL taken as the positive control. Eight compounds were extracted from the H. pinifolia leaves out of which 4-methoxybenzoic acid (4-MBA) at 100 μg/mL showed the maximum inhibition of cell density. 62.5 μg/mL was found to be the maximum biofilm inhibitory concentration of 4-MBA in contrast to which the positive control showed inhibition at 31.2 μg/mL concentration and hence 4-MBA was taken for further evaluation. 4-MBA at 62.5 μg/mL has showed an inhibition of various virulence factors such as inhibition of lasB, protease, pyocyanin formation, rhamnolipid, alginate and chitinase by 87.5%, 83.29%, 91.46%, 79.38%, 86% and 72.09%, respectively. The expression of virulence genes was downregulated the transcript levels by 1.9, 1.63, 2.38 and 1.06 for lasI, lasR, rhlI and rhlR, respectively [124,125,126].
Liu et al., investigated the quorum sensing inhibition and virulence effect of Tea polyphenols (TPs) of Klebsiella pneumoniae. The sub-MICs TPs (p < 0.05) at 100 μg/mL showed 26.28% of violacein inhibition and at 200 μg/mL 56.73% inhibition in comparison with the positive control. The proteolytic activity in untreated bacteria reduced till 100% whereas in TPs treated K4 culture were 31.24% in 200 μg/mL and 16.73% in 400 μg/mL. The EPS production was reduced by 29.24% in 200 μg/mL and 36.79% in 400 μg/mL. The biofilm formation was reduced by 23.7% in 200 μg/mL and 44.4% in 400 μg/mL. This shows that TPs activity is based on its concentration [127,128,129,130].
Chang et al., reported the Anti-quorum sensing activity of marine fungal strain P. chrysogenum DXY-1. There was a reduction in violacein production of C. violaceum CV026 by 53.5% when treated with 0.5 mg/mL of tyrosol. The other virulence factors also showed an inhibition of pyocyanin by 63.3%, elastase activity by 57.8% and proteolytic activity by 9.9% and also showed a significant decrease in the biofilm formation when evaluated against P. aeruginosa PAO1 [101, 131,132,133].
Conclusion
Quorum sensing is a fundamental mechanism in order to regulate various functions, this has also become one of the causes leading to antimicrobial resistance. Quorum Sensing Inhibitors interfere by reducing or suppressing this bacterial communication by various mechanisms hence hinder the bacterial infections. This review summarized different synthetic and natural quorum sensing inhibiting compounds against both Gram-positive and gram-negative bacteria and thus inhibiting different virulence factor of the organism. The different technique depicts broad spectrum of strategies to inhibit the quorum sensing between bacterial cells. The compounds also exhibited their concentration dependent activity against the quorum sensing system of bacteria. Hence discovering newer quorum quenching compounds can be considered as one of the future approaches to tackle the antimicrobial resistance.
Availability of data and materials
Not applicable.
Abbreviations
- QS:
-
Quorum Sensing
- QSI:
-
Quorum Sensing Inhibitors
- AIs:
-
Auto-Inducers
- QQ:
-
Quorum Quenching
- LCD:
-
Low Cell Density
- HCD:
-
High Cell Density
- AIPs:
-
Autoinducing Peptides
- AHL:
-
N-Acyl-homoserine Lactone
- ABC:
-
ATP-binding cassette
- GABA:
-
Gamma-aminobutyric acid
- AMPT:
-
5-Acetyl-4-methyl-2-(3-pyridyl) thiazole
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- C. violaceum :
-
Chromobacterium violaceum
- S. aureus :
-
Staphylococcus aureus
- P. aeruginosa :
-
Pseudomonas aeruginosa
- 5-hydroxymethylfurfural:
-
5-HMF
- 3-oxo-C12-AHL:
-
3-Oxododecanoyl-L-homoserine
- C4-HSL:
-
Lactone N-butanoyl homoserine lactone
- C. elegans :
-
Caenorhabditis elegans
- MIC:
-
Minimum Inhibitory Concentration
- A. baumannii :
-
Acinetobacter baumannii
- PAO1:
-
Pseudomonas aeruginosa O1
- CV026:
-
Chromobacterium violaceum 026
- OD:
-
Optical density
- TPP:
-
Sodium tripolyphosphate
- GNP:
-
Genipin
- PC-NPs:
-
Pre-Crosslinked Nanoparticles
- PC-A:
-
Prototype A
- PC-B:
-
Prototype B
- E. coli :
-
Escherichia coli
- CD:
-
Cyclodextrin
- A. fischeri :
-
Aliivibrio fischeri
- ACD:
-
α-Cyclodextrins
- BCD:
-
ß-cyclodextrins
- GCD:
-
γ-Cyclodextrins
- HPACD:
-
2-Hydroxypropyl α-cyclodextrins
- HPBCD:
-
2-Hydroxypropyl ß-cyclodextrins
- QAACD:
-
Trimethylaminopropyl α-cyclodextrins
- RAMEA:
-
Random methylated α-cyclodextrins
- RAMEB:
-
Random methylated ß-cyclodextrins
- RAMEG:
-
Random methylated γ-cyclodextrins
- SBEBCD:
-
Sulfobutyl ether ß-cyclodextrins
- ACDPS:
-
Epichlorohydrin-crosslinked polymer of α-cyclodextrins
- BCDPS:
-
Epichlorohydrin-crosslinked polymer of ß-cyclodextrins
- EC:
-
Effective concentration
- A. tumefaciens :
-
Agrobacterium tumefaciens
- B. subtilis :
-
Bacillus subtilis
- S. pneumoniae :
-
Streptococcus pneumoniae
- S. epidermidis :
-
Staphylococcus epidermidis
- CLA:
-
Clarithromycin
- CIP:
-
Ciprofloxacin
- IC50:
-
Half maximal Inhibitory Concentration
- R 2 :
-
Correlation Coefficient
- NA:
-
Nalidixic acid
- IV:
-
Oxidovanadium
- V-NA:
-
Vanadium and Nalidixic acid
- EuNPs:
-
Eudragit nanoparticles
- NLCs:
-
Nanostructured lipid carriers
- B. cereus :
-
Bacillus cereus
- GFP:
-
Green Fluorescent Protein
- FIC:
-
Fractional Inhibitory Concentration
- FICI:
-
Fractional Inhibitory Concentration Index
- C. albicans :
-
Candida albicans
- K. pneumoniae :
-
Klebsiella pneumoniae
- DMSO:
-
Dimethylsulfoxide
- btmpp:
-
2,6-Bis(3,4,5-trimethylpyrazolyl)pyridine)
- [Co(btmpp)(NCO)2]:
-
Complex I
- [Co(btmpp)(NCSe)2]:
-
Complex II
- S. sonnei :
-
Shigella sonnei
- Y. enterocolitica :
-
Yersinia enterocolitica
- S. typhimurium :
-
Salmonella typhimurium
- TGK:
-
Substituted γ-lactams
- NiO NPs:
-
Nickel oxide nanoparticles
- A. sobria :
-
Aeromonas sobria
- V. harveyi :
-
Vibrio harveyi
- UDA:
-
Undecanoic Acid
- IAA:
-
Indole-3-Acetic Acid
- IBA:
-
Indole-3-butyric acid
- S. alfredii :
-
Sedum alfredii
- gfp-lasI:
-
Green fluorescence reporter
- ΔrhlI:
-
Rhl QS mutant strain
- μM:
-
Micrometre
- EPS:
-
Extracellular polymeric substance
- APDT:
-
Antimicrobial Photodynamic Therapy
- PS:
-
Photosensitizer
- J/cm2 :
-
Joule per cm square
- ELF:
-
Endolichenic Fungi
- MELF:
-
Metabolites Extract
- AZM:
-
Azithromycin
- H. karajensis :
-
Halobacillus karajensis
- MMS:
-
Methanol/Chloroform Supernatant extract
- kD:
-
Killodalton
- L. nobilis :
-
Laurus nobilis
- HE:
-
N-hexane
- CE:
-
Chloroform
- EAE:
-
Ethyl acetate
- ME:
-
Methanol
- TME:
-
Total methanol extract
- CA:
-
Trans-cinnamaldehyde
- SA:
-
Salicylic acid
- C. tinctoria :
-
Coreopsis tinctoria
- MeOH:
-
Methanol
- L. monocytogenes :
-
Listeria monocytogenes
- P. betle :
-
Piper betle
- CE:
-
Ethanolic Extract
- CA:
-
Crude Alkaloids
- P. fluorescens :
-
Pseudomonas fluorescens
- S. baltica :
-
Shewanella baltica
- CMP:
-
Cytosine monophosphate
- AMP:
-
Adenosine monophosphate
- DKP:
-
Diketopiperazine
- H. pinifolia :
-
Halodule pinifolia
- 4-MBA:
-
4-Methoxybenzoic acid
- TPs:
-
Tea polyphenols
- K4:
-
Cultured K. pneumoniae
- 3-BrBAHSL:
-
(S)-2-(3-bromophenyl)-N-(2-oxotetrahydrofran-3-yl)acetamide
References
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31:224–245. https://doi.org/10.1016/j.biotechadv.2012.10.004
Ciofu O, Giwercman B, Høiby N, Pedersen SS (1994) Development of antibiotic resistance in Pseudomonas aeruginosa during two decades of antipseudomonal treatment at the Danish CF Center. APMIS 102:674–680. https://doi.org/10.1111/j.1699-0463.1994.tb05219.x
Musk D Jr, Hergenrother P (2006) Chemical countermeasures for the control of bacterial biofilms: effective compounds and promising targets. Curr Med Chem 13:2163–2177. https://doi.org/10.2174/092986706777935212
Lewis K (2007) Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. https://doi.org/10.1038/nrmicro1557
Antunes LCM, Ferreira RBR, Buckner MMC, Finlay BB (2010) Quorum sensing in bacterial virulence. Microbiology 156:2271–2282. https://doi.org/10.1099/mic.0.038794-0
Bramhachari PV, Yugandhar NM, Prathyusha AMVN et al (2018) Quorum sensing regulated swarming motility and migratory behavior in bacteria. In: Bramhachari PV (ed) Implication of quorum sensing system in biofilm formation and virulence. Springer, Singapore, pp 49–66
Li J, Chen J, Vidal JE, McClane BA (2011) The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and Beta2 toxin by clostridium perfringens type a non-food-borne human gastrointestinal disease strain F5603. Infect Immun 79:2451–2459. https://doi.org/10.1128/IAI.00169-11
Popham DL, Stevens AM (2006) Bacterial quorum sensing and bioluminescence. Association for Biology Laboratory Education, vol 27, pp 201–215. ISBN 1-890444-09-X
Preda VG, Săndulescu O (2019) Communication is the key: biofilms, quorum sensing, formation and prevention. Discoveries 7:e100. https://doi.org/10.15190/d.2019.13
Zhang L-H, Dong Y-H (2004) Quorum sensing and signal interference: diverse implications: signal interference. Mol Microbiol 53:1563–1571. https://doi.org/10.1111/j.1365-2958.2004.04234.x
Tripathi S, Chandra R, Purchase D et al (2022) Quorum sensing—a promising tool for degradation of industrial waste containing persistent organic pollutants. Environ Pollut 292:118342. https://doi.org/10.1016/j.envpol.2021.118342
Zhou L, Zhang Y, Ge Y et al (2020) Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front Microbiol. https://doi.org/10.3389/fmicb.2020.589640
Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2:a012427. https://doi.org/10.1101/cshperspect.a012427
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77:73–111. https://doi.org/10.1128/MMBR.00046-12
Haque S, Yadav DK, Bisht SC et al (2019) Quorum sensing pathways in Gram-positive and -negative bacteria: potential of their interruption in abating drug resistance. J Chemother 31:161–187. https://doi.org/10.1080/1120009X.2019.1599175
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbio 55:165–199. https://doi.org/10.1146/annurev.micro.55.1.165
Rémy B, Mion S, Plener L et al (2018) Interference in bacterial quorum sensing: a biopharmaceutical perspective. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00203
Jiang Q, Chen J, Yang C et al (2019) Quorum sensing: a prospective therapeutic target for bacterial diseases. BioMed Res Int 2019:e2015978. https://doi.org/10.1155/2019/2015978
Zhao X, Yu Z, Ding T (2020) Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 8:425. https://doi.org/10.3390/microorganisms8030425
Lade H, Paul D, Kweon JH (2014) Quorum quenching mediated approaches for control of membrane biofouling. Int J Biol Sci 10:550–565. https://doi.org/10.7150/ijbs.9028
Rampioni G, Leoni L, Williams P (2014) The art of antibacterial warfare: Deception through interference with quorum sensing–mediated communication. Bioorganic Chem 55:60–68. https://doi.org/10.1016/j.bioorg.2014.04.005
Ni N, Li M, Wang J, Wang B (2009) Inhibitors and antagonists of bacterial quorum sensing. Med Res Rev 29:65–124. https://doi.org/10.1002/med.20145
Hentzer M, Givskov M (2003) Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Investig 112:1300–1307. https://doi.org/10.1172/JCI20074
Rasmussen TB, Givskov M (2006) Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol 296:149–161. https://doi.org/10.1016/j.ijmm.2006.02.005
Dong Y-H, Xu J-L, Li X-Z, Zhang L-H (2000) AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci 97:3526–3531. https://doi.org/10.1073/pnas.97.7.3526
Zhang H-B, Wang L-H, Zhang L-H (2002) Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc Natl Acad Sci 99:4638–4643. https://doi.org/10.1073/pnas.022056699
Zhang H-B, Wang C, Zhang L-H (2004) The quormone degradation system of Agrobacterium tumefaciens is regulated by starvation signal and stress alarmone (p)ppGpp: (p)ppGpp regulates quormone degradation. Mol Microbiol 52:1389–1401. https://doi.org/10.1111/j.1365-2958.2004.04061.x
Huang JJ, Han J-I, Zhang L-H, Leadbetter JR (2003) Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl Environ Microbiol 69:5941–5949. https://doi.org/10.1128/AEM.69.10.5941-5949.2003
Ibrahim YM, Abouwarda AM, Nasr T et al (2020) Antibacterial and anti-quorum sensing activities of a substituted thiazole derivative against methicillin-resistant Staphylococcus aureus and other multidrug-resistant bacteria. Microb Pathog 149:104500. https://doi.org/10.1016/j.micpath.2020.104500
Burt SA, Ojo-Fakunle VTA, Woertman J, Veldhuizen EJA (2014) The Natural antimicrobial carvacrol inhibits quorum sensing in chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 9:e93414. https://doi.org/10.1371/journal.pone.0093414
Gordon RJ, Lowy FD (2008) Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:S350–S359. https://doi.org/10.1086/533591
Rajkumari J, Borkotoky S, Reddy D et al (2019) Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PAO1: insights from in vitro, in vivo and in silico studies. Microbiol Res 226:19–26. https://doi.org/10.1016/j.micres.2019.05.001
Chatterjee M, Anju CP, Biswas L et al (2016) Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol 306:48–58. https://doi.org/10.1016/j.ijmm.2015.11.004
Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. https://doi.org/10.1007/s12038-013-9385-9. Accessed 29 Mar 2023
Seleem NM, Atallah H, Abd El Latif HK et al (2021) Could the analgesic drugs, paracetamol and indomethacin, function as quorum sensing inhibitors? Microb Pathog 158:105097. https://doi.org/10.1016/j.micpath.2021.105097
Stepanović S, Vuković D, Hola V et al (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115:891–899. https://doi.org/10.1111/j.1600-0463.2007.apm_630.x
Seleem NM, Abd El Latif HK, Shaldam MA, El-Ganiny A (2020) Drugs with new lease of life as quorum sensing inhibitors: for combating MDR Acinetobacter baumannii infections. Eur J Clin Microbiol Infect Dis 39:1687–1702. https://doi.org/10.1007/s10096-020-03882-z
Vila-Sanjurjo C, Hembach L, Netzer J et al (2020) Covalently and ionically, dually crosslinked chitosan nanoparticles block quorum sensing and affect bacterial cell growth on a cell-density dependent manner. J Colloid Interface Sci 578:171–183. https://doi.org/10.1016/j.jcis.2020.05.075
Moura MJ, Faneca H, Lima MP et al (2011) In situ forming chitosan hydrogels prepared via ionic/covalent co-cross-linking. Biomacromol 12:3275–3284. https://doi.org/10.1021/bm200731x
Vila-Sanjurjo C, David L, Remuñán-López C et al (2019) Effect of the ultrastructure of chitosan nanoparticles in colloidal stability, quorum quenching and antibacterial activities. J Colloid Interface Sci 556:592–605. https://doi.org/10.1016/j.jcis.2019.08.061
Molnár M, Fenyvesi É, Berkl Z et al (2021) Cyclodextrin-mediated quorum quenching in the Aliivibrio fischeri bioluminescence model system—modulation of bacterial communication. Int J Pharm 594:120150. https://doi.org/10.1016/j.ijpharm.2020.120150
Okano C, Nasuno E, Iimura K, Kato N (2016) Cyclodextrin-immobilized microspheres for uptake of the quorum-sensing signaling molecule N-acylhomoserine lactone. J Appl Polym Sci. https://doi.org/10.1002/app.43198
Kato N, Tanaka T, Nakagawa S et al (2007) Control of virulence factor expression in opportunistic pathogens using cyclodextrin immobilized gel. J Incl Phenom Macrocycl Chem 57:419–423. https://doi.org/10.1007/s10847-006-9228-5
Liu Z, Zhang P, Qin Y et al (2020) Design and synthesis of aryl-substituted pyrrolidone derivatives as quorum sensing inhibitors. Bioorg Chem 105:104376. https://doi.org/10.1016/j.bioorg.2020.104376
Han Y, Hou S, Simon KA et al (2008) Identifying the important structural elements of brominated furanones for inhibiting biofilm formation by Escherichia coli. Bioorg Med Chem Lett 18:1006–1010. https://doi.org/10.1016/j.bmcl.2007.12.032
Manefield M, Rasmussen TB, Henzter M et al (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148:1119–1127. https://doi.org/10.1099/00221287-148-4-1119
Bueloni B, Sanna D, Garribba E et al (2020) Design of nalidixic acid-vanadium complex loaded into chitosan hybrid nanoparticles as smart strategy to inhibit bacterial growth and quorum sensing. Int J Biol Macromol 161:1568–1580. https://doi.org/10.1016/j.ijbiomac.2020.07.304
Sanna D, Ugone V, Sciortino G et al (2018) V IV O complexes with antibacterial quinolone ligands and their interaction with serum proteins. Dalton Trans 47:2164–2182. https://doi.org/10.1039/C7DT04216G
Nair R, Kumar AC, Priya VK et al (2012) Formulation and evaluation of chitosan solid lipid nanoparticles of carbamazepine. Lipids Health Dis 11:72. https://doi.org/10.1186/1476-511X-11-72
Chang Y, Wang P-C, Ma H-M et al (2019) Design, synthesis and evaluation of halogenated furanone derivatives as quorum sensing inhibitors in Pseudomonas aeruginosa. Eur J Pharm Sci 140:105058. https://doi.org/10.1016/j.ejps.2019.105058
Chan YY, Chua KL (2005) The Burkholderia pseudomallei BpeAB-OprB efflux pump: expression and impact on quorum sensing and virulence. J Bacteriol 187:4707–4719. https://doi.org/10.1128/JB.187.14.4707-4719.2005
Abdiali A, Mohammadimehr M, Aghaalaei Y (2006) Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int J Antimicrob Agents 27:196–200. https://doi.org/10.1016/j.ijantimicag.2005.10.007
Jiang K, Yan X, Yu J et al (2020) Design, synthesis, and biological evaluation of 3-amino-2-oxazolidinone derivatives as potent quorum-sensing inhibitors of Pseudomonas aeruginosa PAO1. Eur J Med Chem 194:112252. https://doi.org/10.1016/j.ejmech.2020.112252
Amrit FRG, Ratnappan R, Keith SA, Ghazi A (2014) The C. elegans lifespan assay toolkit. Methods 68:465–475. https://doi.org/10.1016/j.ymeth.2014.04.002
Brackman G, Risseeuw M, Celen S et al (2012) Synthesis and evaluation of the quorum sensing inhibitory effect of substituted triazolyldihydrofuranones. Bioorg Med Chem 20:4737–4743. https://doi.org/10.1016/j.bmc.2012.06.009
Al Marjani MF, Ali FS, Authman SH et al (2020) Identification of novel 1, 3-oxazole and imidazole-5-one that inhibits bacterial biofilm formation of Acinetobacter baumannii. Gene Rep 20:100782. https://doi.org/10.1016/j.genrep.2020.100782
Badmasti F, Siadat SD, Bouzari S et al (2015) Molecular detection of genes related to biofilm formation in multidrug-resistant Acinetobacter baumannii isolated from clinical settings. J Med Microbiol 64:559–564. https://doi.org/10.1099/jmm.0.000058
Baig U, Ansari MA, Gondal MA et al (2020) Single step production of high-purity copper oxide-titanium dioxide nanocomposites and their effective antibacterial and anti-biofilm activity against drug-resistant bacteria. Mater Sci Eng C 113:110992. https://doi.org/10.1016/j.msec.2020.110992
Yang G, Cheng C, Xu G-B et al (2020) Synthesis and antibiofilm evaluation of 3-hydroxy-2,3-dihydroquinazolin-4(1H)-one derivatives against opportunistic pathogen Acinetobacter baumannii. Bioorg Med Chem 28:115606. https://doi.org/10.1016/j.bmc.2020.115606
Bhattacharyya P, Gurung J, Khyriem AB et al (2013) Association of biofilm production with multidrug resistance among clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa from intensive care unit. Indian J Crit Care Med 17:214–218. https://doi.org/10.4103/0972-5229.118416
El-Khouly OA, Henen MA, El-Sayed MA-A et al (2021) Synthesis, anticancer and antimicrobial evaluation of new benzofuran based derivatives: PI3K inhibition, quorum sensing and molecular modeling study. Bioorg Med Chem 31:115976. https://doi.org/10.1016/j.bmc.2020.115976
Coşkun D, Tekin S, Sandal S, Coşkun MF (2016) Synthesis, characterization, and anticancer activity of new benzofuran substituted chalcones. J Chem 2016:1–8. https://doi.org/10.1155/2016/7678486
Cao S-L, Han Y, Yuan C-Z et al (2013) Synthesis and antiproliferative activity of 4-substituted-piperazine-1-carbodithioate derivatives of 2,4-diaminoquinazoline. Eur J Med Chem 64:401–409. https://doi.org/10.1016/j.ejmech.2013.04.017
Nagasundaram N, Govindhan C, Sumitha S et al (2022) Synthesis, characterization and biological evaluation of novel azo fused 2,3-dihydro-1H-perimidine derivatives: In vitro antibacterial, antibiofilm, anti-quorum sensing, DFT, in silico ADME and Molecular docking studies. J Mol Struct 1248:131437. https://doi.org/10.1016/j.molstruc.2021.131437
Pirrung MC (2006) Acceleration of organic reactions through aqueous solvent effects. Chem Eur J 12:1312–1317. https://doi.org/10.1002/chem.200500959
Abinaya M, Gayathri M (2019) Inhibition of biofilm formation, quorum sensing activity and molecular docking study of isolated 3, 5, 7-Trihydroxyflavone from Alstonia scholaris leaf against P. aeruginosa. Bioorg Chem 87:291–301. https://doi.org/10.1016/j.bioorg.2019.03.050
Hopa C, Kara H, Aybey A (2020) Synthesis, structural characterization and biological evaluation of novel mixed-ligand Co(II) complexes as quorum sensing inhibitory agent. J Mol Struct 1202:127322. https://doi.org/10.1016/j.molstruc.2019.127322
Jee J-E, Kwak C-H (2013) Dimeric Ni(II)2 and polymeric Ni(II)4Fe(II) type complexes bridged with Cl− and CN− ligands: X-ray structures and magnetic properties of a dimeric complex of [(tren)Ni(μ-Cl)2Ni(tren)](ClO4)2 and a polymeric complex of {[Fe(CN)6][Ni(tren)]2[Ni(tren)(H2O)]2}Cl2(ClO4)2·4H2O. Inorg Chem Commun 33:95–98. https://doi.org/10.1016/j.inoche.2013.04.014
de Kievit TR (2009) Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol 11:279–288. https://doi.org/10.1111/j.1462-2920.2008.01792.x
Qin X, Thota GK, Singh R et al (2020) Synthetic homoserine lactone analogues as antagonists of bacterial quorum sensing. Bioorg Chem 98:103698. https://doi.org/10.1016/j.bioorg.2020.103698
Kumar Thota G, Tamilarasan D, Balamurugan R (2017) Synthesis of highly functionalized pyrrolidine derivatives from easily accessible diethyl (E)-4-oxohex-2-enedioate: synthesis of highly functionalized pyrrolidine derivatives from easily accessible diethyl (E)-4-oxohex-2-enedioate. Eur J Org Chem 2017:6417–6426. https://doi.org/10.1002/ejoc.201700997
Omwenga EO, Hensel A, Shitandi A, Goycoolea FM (2018) Chitosan nanoencapsulation of flavonoids enhances their quorum sensing and biofilm formation inhibitory activities against an E. coli Top 10 biosensor. Colloids Surf B Biointerfaces 164:125–133. https://doi.org/10.1016/j.colsurfb.2018.01.019
Maruthupandy M, Rajivgandhi GN, Quero F, Li W-J (2020) Anti-quorum sensing and anti-biofilm activity of nickel oxide nanoparticles against Pseudomonas aeruginosa. J Environ Chem Eng 8:104533. https://doi.org/10.1016/j.jece.2020.104533
Rajkumari J, Borkotoky S, Murali A et al (2018) Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb Pathog 118:48–60. https://doi.org/10.1016/j.micpath.2018.03.012
Alipour M, Suntres ZE, Lafrenie RM, Omri A (2010) Attenuation of Pseudomonas aeruginosa virulence factors and biofilms by co-encapsulation of bismuth-ethanedithiol with tobramycin in liposomes. J Antimicrob Chemother 65:684–693. https://doi.org/10.1093/jac/dkq036
Li T, Sun X, Chen H et al (2020) Methyl anthranilate: a novel quorum sensing inhibitor and anti-biofilm agent against Aeromonas sobria. Food Microbiol 86:103356. https://doi.org/10.1016/j.fm.2019.103356
Gutierrez-Pacheco MM, Gonzalez-Aguilar GA, Martinez-Tellez MA et al (2018) Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 89:210–218. https://doi.org/10.1016/j.foodcont.2018.02.007
Diao W-R, Hu Q-P, Feng S-S et al (2013) Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J Agric Food Chem 61:6044–6049. https://doi.org/10.1021/jf4007856
Salini R, Santhakumari S, Veera Ravi A, Karutha Pandian S (2019) Synergistic antibiofilm efficacy of undecanoic acid and auxins against quorum sensing mediated biofilm formation of luminescent Vibrio harveyi. Aquaculture 498:162–170. https://doi.org/10.1016/j.aquaculture.2018.08.038
You J, Xue X, Cao L et al (2007) Inhibition of Vibrio biofilm formation by a marine actinomycete strain A66. Appl Microbiol Biotechnol 76:1137–1144. https://doi.org/10.1007/s00253-007-1074-x
Zhu H, He C-C, Chu Q-H (2011) Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular: quorum-sensing inhibition by Auricularia auricular pigments. Lett Appl Microbiol 52:269–274. https://doi.org/10.1111/j.1472-765X.2010.02993.x
Saqr AA, Aldawsari MF, Khafagy E-S et al (2021) A novel use of allopurinol as a quorum-sensing inhibitor in Pseudomonas aeruginosa. Antibiotics 10:1385. https://doi.org/10.3390/antibiotics10111385
Bhattacharjee A, Nusca TD, Hochbaum AI (2016) Rhamnolipids mediate an interspecies biofilm dispersal signaling pathway. ACS Chem Biol 11:3068–3076. https://doi.org/10.1021/acschembio.6b00750
Hema M, Vasudevan S, Balamurugan P, Adline Princy S (2017) Modulating the global response regulator, LuxO of V. cholerae quorum sensing system using a pyrazine dicarboxylic acid derivative (PDCApy): an antivirulence approach. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2017.00441
Zhu M, Yang Y, Wang M et al (2021) A deep insight into the suppression mechanism of Sedum alfredii root exudates on Pseudomonas aeruginosa based on quorum sensing. Ecotoxicol Environ Saf 217:112240. https://doi.org/10.1016/j.ecoenv.2021.112240
Miladi H, Zmantar T, Chaabouni Y et al (2016) Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb Pathog 99:95–100. https://doi.org/10.1016/j.micpath.2016.08.008
Casilag F, Lorenz A, Krueger J et al (2016) The LasB elastase of Pseudomonas aeruginosa acts in concert with alkaline protease AprA to prevent flagellin-mediated immune recognition. Infect Immun 84:162–171. https://doi.org/10.1128/IAI.00939-15
Geng YF, Yang C, Zhang Y et al (2021) An innovative role for luteolin as a natural quorum sensing inhibitor in Pseudomonas aeruginosa. Life Sci 274:119325. https://doi.org/10.1016/j.lfs.2021.119325
Zhang Y, Kong J, Huang F et al (2018) Hexanal as a QS inhibitor of extracellular enzyme activity of Erwinia carotovora and Pseudomonas fluorescens and its application in vegetables. Food Chem 255:1–7. https://doi.org/10.1016/j.foodchem.2018.02.038
Dieltjens L, Appermans K, Lissens M et al (2020) Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat Commun 11:107. https://doi.org/10.1038/s41467-019-13660-x
Shukla A, Parmar P, Patel B et al (2021) Breaking bad: Better call gingerol for improving antibiotic susceptibility of Pseudomonas aeruginosa by inhibiting multiple quorum sensing pathways. Microbiol Res 252:126863. https://doi.org/10.1016/j.micres.2021.126863
Krivov GG, Shapovalov MV, Dunbrack RL (2009) Improved prediction of protein side-chain conformations with SCWRL4: side-chain prediction with SCWRL4. Proteins Struct Funct Bioinforma 77:778–795. https://doi.org/10.1002/prot.22488
Shukla A, Mehta K, Parmar J et al (2019) Depicting the exemplary knowledge of microbial exopolysaccharides in a nutshell. Eur Polym J 119:298–310. https://doi.org/10.1016/j.eurpolymj.2019.07.044
Abdulrahman H, Misba L, Ahmad S, Khan AU (2020) Curcumin induced photodynamic therapy mediated suppression of quorum sensing pathway of Pseudomonas aeruginosa: an approach to inhibit biofilm in vitro. Photodiagn Photodyn Ther 30:101645. https://doi.org/10.1016/j.pdpdt.2019.101645
Islam B, Khan SN, Haque I et al (2008) Novel anti-adherence activity of mulberry leaves: inhibition of Streptococcus mutans biofilm by 1-deoxynojirimycin isolated from Morus alba. J Antimicrob Chemother 62:751–757. https://doi.org/10.1093/jac/dkn253
Vassena C, Fenu S, Giuliani F et al (2014) Photodynamic antibacterial and antibiofilm activity of RLP068/Cl against Staphylococcus aureus and Pseudomonas aeruginosa forming biofilms on prosthetic material. Int J Antimicrob Agents 44:47–55. https://doi.org/10.1016/j.ijantimicag.2014.03.012
Prateeksha BR, Yusuf MA et al (2020) Endolichenic fungus, Aspergillus quandricinctus of Usnea longissima inhibits quorum sensing and biofilm formation of Pseudomonas aeruginosa PAO1. Microb Pathog 140:103933. https://doi.org/10.1016/j.micpath.2019.103933
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900. https://doi.org/10.1128/jb.172.2.884-900.1990
Caballero AR, Moreau JM, Engel LS et al (2001) Pseudomonas aeruginosa protease IV enzyme assays and comparison to other pseudomonas proteases. Anal Biochem 290:330–337. https://doi.org/10.1006/abio.2001.4999
Chang A, He Q, Li L et al (2021) Exploring the quorum sensing inhibition of isolated chrysin from Penicillium chrysogenum DXY-1. Bioorg Chem 111:104894. https://doi.org/10.1016/j.bioorg.2021.104894
Chang A, Sun S, Li L et al (2019) Tyrosol from marine Fungi, a novel Quorum sensing inhibitor against Chromobacterium violaceum and Pseudomonas aeruginosa. Bioorg Chem 91:103140. https://doi.org/10.1016/j.bioorg.2019.103140
Serra R, Grande R, Butrico L et al (2015) Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther 13:605–613. https://doi.org/10.1586/14787210.2015.1023291
Ghoreishi FS, Roghanian R, Emtiazi G (2020) Inhibition of quorum sensing-controlled virulence factors with natural substances and novel protease, obtained from Halobacillus karajensis. Microb Pathog 149:104555. https://doi.org/10.1016/j.micpath.2020.104555
Wadday AK, Saleh ZA, Al-Marjani MF (2019) Spectroscopic characteristics and energy transfer of bacterial pigment: (pyocyanin/curcumin). Erode, India, p 020014. https://doi.org/10.1063/1.5141438
Zaghian S, Shokri D, Emtiazi G (2012) Co-production of a UV-stable bacteriocin-like inhibitory substance (BLIS) and indole-3-acetic acid hormone (IAA) and their optimization by Taguchi design in Bacillus pumilus. Ann Microbiol 62:1189–1197. https://doi.org/10.1007/s13213-011-0359-6
Inés Molina RD, Campos-Silva R, Díaz MA et al (2020) Laurel extracts inhibit Quorum sensing, virulence factors and biofilm of foodborne pathogens. LWT 134:109899. https://doi.org/10.1016/j.lwt.2020.109899
Luciardi MC, Blázquez MA, Cartagena E et al (2016) Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT Food Sci Technol 68:373–380. https://doi.org/10.1016/j.lwt.2015.12.056
Boulos L, Prévost M, Barbeau B et al (1999) LIVE/DEAD® BacLight™: application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J Microbiol Methods 37:77–86. https://doi.org/10.1016/S0167-7012(99)00048-2
Ahmed SAKS, Rudden M, Smyth TJ et al (2019) Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl Microbiol Biotechnol 103:3521–3535. https://doi.org/10.1007/s00253-019-09618-0
Stover CK, Pham XQ, Erwin AL et al (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. https://doi.org/10.1038/35023079
Elshikh M, Ahmed S, Funston S et al (2016) Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett 38:1015–1019. https://doi.org/10.1007/s10529-016-2079-2
Mu Y, Zeng H, Chen W (2020) Okanin in Coreopsis tinctoria Nutt is a major quorum-sensing inhibitor against Chromobacterium violaceum. J Ethnopharmacol 260:113017. https://doi.org/10.1016/j.jep.2020.113017
Gemiarto AT, Ninyio NN, Lee SW et al (2015) Isoprenyl caffeate, a major compound in manuka propolis, is a quorum-sensing inhibitor in Chromobacterium violaceum. Antonie Van Leeuwenhoek 108:491–504. https://doi.org/10.1007/s10482-015-0503-6
Ryan KS, Balibar CJ, Turo KE et al (2008) The violacein biosynthetic enzyme VioE shares a fold with lipoprotein transporter proteins. J Biol Chem 283:6467–6475. https://doi.org/10.1074/jbc.M708573200
Wei LN, Shi CZ, Luo CX et al (2020) Phloretin inhibits biofilm formation by affecting quorum sensing under different temperature. LWT 131:109668. https://doi.org/10.1016/j.lwt.2020.109668
Debunne N, Verbeke F, Janssens Y et al (2018) Chromatography of Quorum Sensing Peptides: An Important Functional Class of the Bacterial Peptidome. Chromatographia 81:25–40. https://doi.org/10.1007/s10337-017-3411-2
Phukhamsakda C, Macabeo A, Yuyama K et al (2018) Biofilm Inhibitory Abscisic Acid Derivatives from the Plant-Associated Dothideomycete Fungus. Roussoella sp Molecules 23:2190. https://doi.org/10.3390/molecules23092190
Guzman JPMD, Yatip P, Soowannayan C, Maningas MBB (2022) Piper betle L. leaf extracts inhibit quorum sensing of shrimp pathogen Vibrio harveyi and protect Penaeus vannamei postlarvae against bacterial infection. Aquaculture 547:737452. https://doi.org/10.1016/j.aquaculture.2021.737452
Yatip P, Nitin Chandra Teja D, Flegel TW, Soowannayan C (2018) Extract from the fermented soybean product Natto inhibits Vibrio biofilm formation and reduces shrimp mortality from Vibrio harveyi infection. Fish Shellfish Immunol 72:348–355. https://doi.org/10.1016/j.fsi.2017.11.008
Anetzberger C, Reiger M, Fekete A et al (2012) Autoinducers act as biological timers in Vibrio harveyi. PLoS ONE 7:e48310. https://doi.org/10.1371/journal.pone.0048310
Wang Y, Wang Y, Chen J et al (2021) Screening and preservation application of quorum sensing inhibitors of Pseudomonas fluorescens and Shewanella baltica in seafood products. LWT 149:111749. https://doi.org/10.1016/j.lwt.2021.111749
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. https://doi.org/10.1038/nprot.2007.521
Djordjevic D, Wiedmann M, McLandsborough LA (2002) Microtiter Plate Assay for Assessment of Listeria monocytogenes Biofilm Formation. Appl Environ Microbiol 68:2950–2958. https://doi.org/10.1128/AEM.68.6.2950-2958.2002
Danaraj J, Mariasingarayan Y, Ayyappan S, Karuppiah V (2020) Seagrass Halodule pinifolia active constituent 4-methoxybenzioic acid (4-MBA) inhibits quorum sensing mediated virulence production of Pseudomonas aeruginosa. Microb Pathog 147:104392. https://doi.org/10.1016/j.micpath.2020.104392
Adonizio A, Kong K-F, Mathee K (2008) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother 52:198–203. https://doi.org/10.1128/AAC.00612-07
Kessler E, Safrin M, Olson JC, Ohman DE (1993) Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem 268:7503–7508. https://doi.org/10.1016/S0021-9258(18)53203-8
Liu W, Lu H, Chu X et al (2020) Tea polyphenols inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances resistance to Klebsiella pneumoniae infection in Caenorhabditis elegans model. Microb Pathog 147:104266. https://doi.org/10.1016/j.micpath.2020.104266
Ravn L, Christensen AB, Molin S et al (2001) Methods for detecting acylated homoserine lactones produced by Gram-negative bacteria and their application in studies of AHL-production kinetics. J Microbiol Methods 44:239–251. https://doi.org/10.1016/S0167-7012(01)00217-2
Peng D, Li X, Liu P et al (2018) Transcriptional regulation of galF by RcsAB affects capsular polysaccharide formation in Klebsiella pneumoniae NTUH-K2044. Microbiol Res 216:70–78. https://doi.org/10.1016/j.micres.2018.08.010
Nadar S, Khan T, Patching SG, Omri A (2022) Development of antibiofilm therapeutics strategies to overcome antimicrobial drug resistance. Microorganisms 10:303. https://doi.org/10.3390/microorganisms10020303
McClean KH, Winson MK, Fish L et al (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703–3711. https://doi.org/10.1099/00221287-143-12-3703
Chen G, Swem LR, Swem DL et al (2011) A Strategy for Antagonizing Quorum Sensing. Mol Cell 42:199–209. https://doi.org/10.1016/j.molcel.2011.04.003
Sionov RV, Steinberg D (2022) Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic bacteria. Microorganisms 10:1239. https://doi.org/10.3390/microorganisms10061239
Acknowledgements
The authors would like to acknowledge University of Mumbai for the support to write this review.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SN conceptualized the work, TB wrote the manuscript and ST did the critiqued the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boban, T., Nadar, S. & Tauro, S. Breaking down bacterial communication: a review of quorum quenching agents. Futur J Pharm Sci 9, 77 (2023). https://doi.org/10.1186/s43094-023-00526-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00526-9