Abstract
Background
Infections caused by Klebsiella pneumoniae are common and result in high mortality rates. In vitro studies demonstrated the potency of cefoperazone/sulbactam (CPZ/SUL) against Klebsiella pneumoniae. However, the clinical efficacy of CPZ/SUL for the treatment of K. pneumoniae bacteremia has not been studied.
Objectives
This study aimed to associate the clinical outcomes of patients with bacteremia with the minimal inhibitory concentrations (MICs) of CPZ/SUL against the causative K. pneumoniae isolates.
Methods
This multicenter, retrospective study was conducted in Taiwan between July 2017 and April 2021. Patients with K. pneumoniae bacteremia treated with CPZ/SUL were enrolled in this study. CPZ/SUL MICs were determined using the agar dilution method. Data on the patients’ clinical outcomes and characteristics were collected and analyzed.
Results
In total, 201 patients were enrolled. Among the causative K. pneumoniae isolates, 180 (89.5%) were susceptible to CPZ/SUL. Most patients (n = 156, 77.6%) had favorable outcomes. The 30-day mortality rate was 11.9% (n = 24). Multivariate risk analyses showed that higher APACHE II score (Odds Ratio [OR], 1.14; Confidence Interval [CI], 1.07–1.21; p < 0.001), metastatic tumors (OR, 5.76; CI, 2.31–14.40; p < 0.001), and causative K. pneumoniae CPZ/SUL MICs > 16 µg/ml (OR, 4.30; CI, 1.50–12.27; p = 0.006) were independently associated with unfavorable outcomes.
Conclusion
Patients with K. pneumoniae bacteremia treated with CPZ/SUL at a ratio 1:1 had favorable outcomes when the CPZ/SUL MICs were ≤ 16 µg/ml. Patients with higher APACHE II scores and metastatic tumors had unfavorable outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Klebsiella pneumoniae is a common and life-threatening community-acquired, healthcare-associated, and hospital-acquired pathogen. K. pneumoniae can cause pneumonia, urinary tract infections, intra-abdominal infections, liver abscesses, bacteremia, and other invasive infections [1,2,3]. The mortality rates of patients with K. pneumoniae bacteremia differed from 20 to 50%, depending on the infected population, and the rates became higher when the severity of the infection increased and the presence of carbapenem resistances [4,5,6]. The emergence of extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae during the past decades has hindered the treatment of these infections and further limited available drug choices for antimicrobial therapy [7,8,9]. Adequate treatment of patients infected with this problematic pathogen is a major concern to physicians.
Cefoperazone (CPZ) is a third-generation cephalosporin that is active against the most commonly encountered gram-negative bacteria (GNB) [10,11,12], but not ESBL-producing GNB. The inclusion of sulbactam (SUL), a penicillanic acid sulfone with activity against Ambler class A enzymes, broadened the antimicrobial spectrum of CPZ [13, 14].
Over the past, the emergence of ESBL-producing K. pneumoniae has caused a serious clinical burden [7,8,9]. The Taiwan Surveillance of Antimicrobial Resistance program conducted from 2002 to 2012 reported that the prevalence of ESBL-producing K. pneumoniae increased from 4.8 to 11.9% in Taiwan [15]. According to the SENTRY antimicrobial surveillance program data, the prevalence of multidrug-resistant (MDR) Enterobacterales-associated bloodstream infections increased from 6.2 to 15.8% between 1997 and 2016 [9].
The CPZ/SUL combination is active against many MDR GNBs, including ESBL-producing Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii [16, 17]. CPZ/SUL is effective against MDR GNBs that cause febrile neutropenia, intra-abdominal infections, community-acquired pneumonia, and hospital-acquired pneumonia [12, 18,19,20,21,22]. However, there are no available minimal inhibitory concentration (MIC) interpretation breakpoints for the CPZ/SUL combination according to the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [23, 24]. In Taiwan, antimicrobial susceptibility test reports for CPZ/SUL are generated by automated testing using a 2:1 ratio of CPZ to SUL, and the results are interpreted using the CLSI breakpoints for cefoperazone against Enterobacterales. Studies have reported that CPZ/SUL administered at a 1:1 ratio has superior antibacterial activities against ESBL-producing K. pneumoniae and most MDR GNB compared with the 2:1 ratio [25, 26]. A recent study revealed an 82.7% clinical success rate in treating bacteremia caused by ESBL-producing Enterobacterales with 1:1 CPZ/SUL [27].
We conducted a multicenter, retrospective study to correlate the MIC values of a 1:1 ratio of CPZ/SUL against K. pneumoniae and the clinical outcomes of patients with K. pneumoniae bacteremia.
Materials and methods
Study design and patients
This multicenter study was conducted between July 2017 and September 2022 at eight medical centers located in different parts of Taiwan, including Southern Taiwan (Chi Mei Medical Center [CMMC], Kaohsiung Chang-Guan Memorial Hospital [KCGMH], and Kaohsiung Medical University Hospital [KMUH]), Central Taiwan (China Medical University Hospital [CMUH] and Taichung Veterans General Hospital [TCVGH]), and Northern Taiwan (Linkou Chang Gung Memorial Hospital [LCGMH], Tri-Service General Hospital [TSGH], and Taipei Veterans General Hospital [TVGH]).
The enrolled patients were > 20 years of age had monomicrobial bacteremia caused by K. pneumoniae and were initially treated using antimicrobial monotherapy with a 1:1 ratio of CPZ/SUL within 24 h of bacteremia onset with treatment lasting for more than 72 h. The onset of bacteremia was defined as the date of index blood culture collected [25, 28]. CPZ/SUL was given intravenously every 12 h at a standard dosage of 2/2 g, with dosage modification as the manufacturer’s guidelines, according to the estimated creatinine clearance using the Cockroft–Gault equation [29]. Patients receiving additional antimicrobial therapies exceeding 48 h were excluded, except those treatments were targeting GPCs, virus or fungi. This study was approved by the Institutional Review Board (IRB) of the TSGH (No. 1-106-05-116) and the IRBs of all other participating hospitals.
Antimicrobial susceptibility test
The antimicrobial MICs (µg/ml) were determined using the agar dilution method in accordance with CLSI recommendations [23]. The 1:1 combination ratio was used [25]. CPZ/SUL powder was purchased from TTY Biopharm (Taipei, Taiwan).
Variable definition and assessment of the treatment efficacy
The Charlson Comorbidity Index [30] was used to assess comorbidities. Immunosuppressant therapy indicated those patient receiving prednisolone for at least10mg per day (or equivalent potency agents) from 2 days before bacteremia onset till 30 days post the event. The Acute Physiology and Chronic Health Evaluation II (APACHE II) [31] was used to assess disease severity. The source of bacteremia was classified as respiratory tract infection/pneumonia, urinary tract infection, soft-tissue infections, intraabdominal infection, or primary bloodstream infection, according to the definitions of the Centers for Disease Control and Prevention [32]. Clinical outcomes were assessed at 30 days. The clinical outcomes were recorded and divided into four categories: cure, improvement, lack of efficacy, and death. A cure was defined as the absence of symptoms and signs of infection without the requirement for additional antibiotic therapy and a negative result in the subsequent blood culture within a week of the onset of bacteremia. Improvement indicated that the symptoms and signs subsided with or without laboratory improvement, based on clinical judgment, and further antibiotic treatment was required. Lack of efficacy was defined as clinical progression or persistent bacteremia at the end of CPZ/SUL treatment [33]. Regarding the correlation between treatment efficacy and the MICs of CPZ/SUL, cure and improvement were defined as favorable outcomes. In contrast, a lack of efficacy and death were defined as unfavorable outcomes.
Statistical analyses
Descriptive statistics were used to present the demographic characteristics of enrolled patients. The demographic characteristics of the two groups (MIC ≤ 16 and MIC > 16) were compared using the Fisher’s exact test for categorical variables. Continuous variables are presented as the mean ± standard deviation and compared using Student’s t-test. Statistical significance was set at p < 0.05. A multivariate analysis was performed for all variables that were statistically significant in the univariate analysis. Odds ratios (ORs) with 95% confidence intervals (CIs) and p-values were calculated. All statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA).
Results
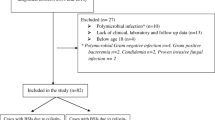
During the study period, 201 patients with K. pneumoniae bacteremia were enrolled based on the patient selection criteria (Fig. 1). The patient demographic data were presented in Table 1. The majority of patients were male. The most prevalent comorbidities included diabetes mellitus, impaired liver function, and impaired renal function. The primary cause of bacteremia was intra-abdominal infection, followed by primary bacteremia, respiratory tract infection, and urinary tract infections. Antimicrobial susceptibility results for K. pneumoniae are detailed in Table 2.
Methodology for application of exclusion criteria. CPZ/SUL, cefoperazone/sulbactam; CGMH-LK, Chang Gung Memorial Hospital; CMMH, Chi Mei Medical Hospital; CMUH, China Medical University Hospital; KCGMH, Kaohsiung Chang Gung Memorial Hospital; KMUH, Kaohsiung Medical University Hospital; TSGH Tri-Service General Hospital; TVGH Taipei Veterans General Hospital; TCTVGH, Taichung Veterans General Hospital
The MIC of CPZ alone and the 1:1 combination of CPZ/SUL against K. pneumoniae are shown in Table 3. The MIC ranges and MIC50 values for CPZ and CPZ/SUL were similar. However, the MIC90 values were lower for CPZ/SUL (MIC90: 32 ug/ml) than for CPZ (MIC90: >64 ug/ml) alone. Among the 201 isolates, 180 (89.55%) were susceptible, six (2.99%) were intermediate, and 15 (7.46%) were resistant to CPZ/SUL. For isolates that were not susceptible to CPZ, the addition of SUL restored the susceptibility rate from 0 to 53.33% and reduced the resistance rate from 82.22 to 33.33% (Table 3). Distribution of the cefoperazone/sulbactam MIC values among those K. pneumoniae isolates were showed in Fig. 2. Most of those K. pneumoniae isolates in this study exhibited MIC values of less than 8 µg/ml (< 8 µg/ml, n = 157, 78.11%) (Fig. 2).
Outcome evaluations revealed that 77.61% exhibited favorable outcomes (cure and improvement) and 22.39% showed unfavorable outcomes (death and lack of treatment efficacy). (Table 1). The clinical outcomes correlated with CPZ/SUL MIC values were showed in Fig. 2. As the MIC value increased, the rate of favorable outcomes decreased, and the 30-days mortality rate increased.
Comparing the patient characteristics and outcomes in causative K. pneumoniae isolates with CPZ/SUL MIC ≤ 16 µg/ml and > 16 µg/ml in Table 1, we observed that unfavorable outcomes were more frequent in those with MIC > 16 µg/ml than those with MIC ≤ 16 µg/ml. Those infected by isolates with MIC > 16 µg/ml had a higher APACHE II scores and higher prevalence of impaired renal function. There were no significant differences in sex, age, or source of infection between the two groups.
Logistic regression analysis of the prognostic factors for unfavorable outcomes was shown in Table 4. Comparing the two groups, patients with higher APACHE II score (mean ± standard deviation, 12.74 ± 6.35 vs. 18.27 ± 7.18 points; p < 0.001), metastatic tumors (n = 19, 12.18% vs. n = 16, 35.56%; p < 0.001), and infection by K. pneumoniae isolates with CPZ/SUL MIC > 16 µg/ml (n = 10, 6.41% vs. n = 11, 24.44%; p = 0.001) were associated with a higher risk of unfavorable outcomes in univariate analysis. In multivariate analysis, patients with a higher APACHE II score (OR, 1.14; 95% CI, 1.07–1.21; p < 0.001), metastatic tumors (OR, 5.76; CI, 2.31–14.40; p < 0.001), and infection by K. pneumoniae isolates with CPZ/SUL MIC > 16 µg/ml (OR, 4.30; CI, 1.50–12.27; p = 0.006) were independently associated with unfavorable outcomes.
Discussion
This is the first multicenter study to investigate the effects of CPZ/SUL therapy in patients with K. pneumoniae bacteremia and to provide reference clinical breakpoints and prognostic factors for outcomes. The correlation analysis of the MIC values of CPZ/SUL against K. pneumoniae with the clinical outcomes revealed that most patients (81.1%) infected by isolates with MIC ≤ 16 µg/ml had favorable outcomes. MIC values > 16 µg/ml were independently associated with unfavorable outcomes. In addition, higher APACHE II scores and metastatic tumors were associated with unfavorable outcomes in patients with K. pneumoniae bacteremia.
The major mechanism underlying third-generation cephalosporin resistance in K. pneumoniae in Taiwan and worldwide is the presence of ESBL genes [34, 35]. The consumption of carbapenems has increased, which has promoted the spread of carbapenem-resistant K. pneumoniae [36, 37]. Therefore, CPZ/SUL may offer a valuable carbapenem-sparing alternative for effectively covering ESBL producers, which is important in antimicrobial stewardship [27]. However, in areas where the prevalence of carbapenem resistance is significant, the empirical use of CPZ/SUL should be approached with caution [38, 39].
The addition of SUL effectively restored the efficacy of CPZ from 77.6 to 89.6%. Even in isolates that were not susceptible to CPZ, the addition of SUL to CPZ restored antimicrobial susceptibility from 0 to 53% in the current study (Table 3). In most previous studies, the ratio of CPZ to SUL was 2:1 [40,41,42,43]. Recent studies have established that CPZ/SUL ratio of 2:1 and 1:1 significantly increased the efficacy against ESBL strains and MDR GNB compared with CPZ alone [25]. Moreover, CPZ/SUL at a 1:1 ratio produced better activity against MDR GNB than that at a 2:1 ratio [26].
To date, no CLSI clinical breakpoints have been reported for CPZ/SUL in K. pneumoniae. The CLSI CPZ breakpoints for Enterobacterales (MIC ≤ 16 µg/ml, susceptible; MIC = 32 µg/ml, intermediate; MIC ≥ 64 µg/ml, resistant) [23] are often used to interpret susceptibility results for CPZ/SUL. Despite the emergence of resistance, CPZ/SUL maintains good antimicrobial efficacy. A large-scale study in China from 2010 to 2018 reported susceptibility rates of K. pneumoniae to CPZ/SUL (2:1) ranging from 72.1 to 76.9% [44]. In the current study, the susceptibility was even higher (89.6%) when using the 1:1 CPZ: SUL combination. CPZ/SUL MIC > 16 µg/ml was associated with unfavorable outcomes, indicating that patients with bacteremia who were infected with K. pneumoniae isolates with MIC > 16 µg/ml should not be treated with CPZ/SUL. In contrast, most patients with CPZ/SUL MIC ≤ 16 µg/ml exhibited favorable outcomes (81.1%), indicating the efficacy of treatment with 1:1 CPZ/SUL.
In the current study, we observed that the APACHE score and the presence of metastatic tumors were significant risk factors for unfavorable outcomes. These findings were consistent with prior investigations on K. pneumoniae bacteremia [45, 46]. Therefore, judicious antimicrobial treatment is crucial for patients with such risk factors. When the MICs ≤ 16 µg/ml, CPZ/SUL could be confidently used to treat K. pneumoniae bacteremia. It is also important to follow the results of antimicrobial susceptibilities and following antimicrobial stewardship principles.
This study had some limitations. The major limitations are its retrospective design with potential intrinsic selection bias, and the fact that the detailed treatment course cannot be controlled. Further randomized controlled studies are required to confirm these findings. The strengths of this study include the inclusion of a relatively large number of patients from multiple medical centers located in representative regions of Taiwan using stringent inclusion criteria. Our findings provide clinicians with useful information regarding the outcomes and risk factors of patients with K. pneumoniae bacteremia treated with CPZ/SUL.
Conclusion
Patients with K. pneumoniae bacteremia treated with CPZ/SUL at a ratio of 1:1 had a favorable outcome when the CPZ/SUL MICs were ≤ 16 µg/ml. Higher APACHE II scores and metastatic tumors were associated with unfavorable outcomes.
Data availability
No datasets were generated or analysed during the current study.
References
Liao CH, Huang YT, Hsueh PR (2022) Multicenter surveillance of capsular serotypes, virulence genes, and antimicrobial susceptibilities of Klebsiella pneumoniae causing bacteremia in Taiwan, 2017–2019. Front Microbiol 13:783523
Magill SS, O’Leary E, Janelle SJ et al (2018) Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 379:1732–1744
Restuccia PA, Cunha BA, Klebsiella (1984) Infect Control 5:343–347
Tumbarello M, Viale P, Viscoli C et al (2012) Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950
Xu L, Sun X, Ma X (2017) Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob 16:18
Siu LK, Yeh KM, Lin JC et al (2012) Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 12:881–887
Rupp ME, Fey PD (2003) Extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: considerations for diagnosis, prevention and drug treatment. Drugs 63:353–365
Paterson DL, Bonomo RA (2005) Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18:657–686
Diekema DJ, Hsueh PR, Mendes RE et al (2019) The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother ; 63
Brogden RN, Carmine A, Heel RC et al (1981) Cefoperazone: a review of its in vitro antimicrobial activity, pharmacological properties and therapeutic efficacy. Drugs 22:423–460
Matsubara N, Minami S, Muraoka T et al (1979) In vitro antibacterial activity of cefoperazone (T-1551), a new semisynthetic cephalosporin. Antimicrob Agents Chemother 16:731–735
Chiang T-T, Tang H-J, Chiu C-H et al (2016) Antimicrobial activities of cefoperazone-sulbactam in comparison to cefoperazone against clinical organisms from medical centers in Taiwan. J Med Sci 36:229
Williams JD (1997) beta-lactamase inhibition and in vitro activity of sulbactam and sulbactam/cefoperazone. Clin Infect Dis 24:494–497
Akova M (2008) Sulbactam-containing beta-lactamase inhibitor combinations. Clin Microbiol Infect 14(Suppl 1):185–188
Lin WP, Wang JT, Chang SC et al (2016) The antimicrobial susceptibility of Klebsiella pneumoniae from community settings in Taiwan, a trend analysis. Sci Rep 6:36280
Wang FD, Lin ML, Lee WS et al (2004) In vitro activities of beta-lactam antibiotics alone and in combination with sulbactam against gram-negative bacteria. Int J Antimicrob Agents 23:590–595
Tseng SH, Lee CM, Lin TY et al (2011) Emergence and spread of multi-drug resistant organisms: think globally and act locally. J Microbiol Immunol Infect 44:157–165
Xia J, Zhang D, Xu Y et al (2014) A retrospective analysis of carbapenem-resistant Acinetobacter baumannii-mediated nosocomial pneumonia and the in vitro therapeutic benefit of cefoperazone/sulbactam. Int J Infect Dis 23:90–93
Guclu E, Kaya G, Ogutlu A et al (2020) The effect of cefoperazone sulbactam and piperacillin tazobactam on mortality in gram-negative nosocomial infections. J Chemother 32:118–123
Chandra A, Dhar P, Dharap S et al (2008) Cefoperazone-sulbactam for treatment of intra-abdominal infections: results from a randomized, parallel group study in India. Surg Infect (Larchmt) 9:367–376
Lan SH, Chang SP, Lai CC et al (2020) Efficacy and safety of cefoperazone-sulbactam in empiric therapy for febrile neutropenia: a systemic review and meta-analysis. Med (Baltim) 99:e19321
Huang CT, Chen CH, Chen WC et al (2022) Clinical effectiveness of cefoperazone-sulbactam vs. piperacillin-tazobactam for the treatment of pneumonia in elderly patients. Int J Antimicrob Agents 59:106491
Weinstein M, Patel J, Bobenchik A, Clinical and laboratory standards institute (2019) Performance standards for antimicrobial susceptibility testing. M 100:148–149
European committee on antimicrobial susceptibility testing (2018) Breakpoint tables for interpretation of MICs and zone diameters. version 9
Chang PC, Chen CC, Lu YC et al (2018) The impact of inoculum size on the activity of cefoperazone-sulbactam against multidrug resistant organisms. J Microbiol Immunol Infect 51:207–213
Lai CC, Chen CC, Lu YC et al (2018) Appropriate composites of cefoperazone-sulbactam against multidrug-resistant organisms. Infect Drug Resist 11:1441–1445
Chen RZ, Lu PL, Yang TY et al (2024) Efficacy of cefoperazone/sulbactam for ESBL-producing Escherichia coli and Klebsiella pneumoniae bacteraemia and the factors associated with poor outcomes. J Antimicrob Chemother 793:648–655
Lee YT, Chiang MC, Kuo SC et al (2016) Carbapenem breakpoints for Acinetobacter baumannii group: supporting clinical outcome data from patients with bacteremia. PLoS ONE 11:e0163271
Rho JP, Castle S, Smith K et al (1992) Effect of impaired renal function on the pharmacokinetics of coadministered cefoperazone and sulbactam. J Antimicrob Chemother 29:701–709
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Knaus WA, Draper EA, Wagner DP et al (1985) APACHII: a severity of disease calssification system. Crit Care Med 13(10):818–829
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332
Nguyen M, Eschenauer GA, Bryan M et al (2010) Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis 67(2):180–184
Juan CH, Chou SH, Chen IR et al (2022) Intestinal colonisation with hypervirulent or third-generation cephalosporin-resistant Klebsiella pneumoniae strains upon hospital admission in a general ward in Taiwan. Int J Antimicrob Agents 60:106624
Castanheira M, Simner PJ, Bradford PA (2021) Extended-spectrum beta-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob Resist 3:dlab092
Van Boeckel TP, Gandra S, Ashok A et al (2014) Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14:742–750
Armand-Lefevre L, Angebault C, Barbier F et al (2013) Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 57:1488–1495
van Duin D, Doi Y (2017) The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8(4):460–469
Lee CR, Lee JH, Park KS et al (2016) Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol 7:184902
Hung MN, Hsueh PR, Chang HT et al (2007) In vitro activities of various piperacillin and sulbactam combinations against bacterial pathogens isolated from intensive care units in Taiwan: SMART 2004 programme data. Int J Antimicrob Agents 29:145–152
Knapp CC, Sierra-Madero J, Washington JA (1990) Comparative in vitro activity of cefoperazone and various combinations of cefoperazone/sulbactam. Diagn Microbiol Infect Dis 13:45–49
Gelfand MS, Grogan JT, Haas MJ (1989) In vitro comparison of cefoperazone/sulbactam with selected antimicrobials against 300 bacteroides isolates. Inhibitory activity and time-kill kinetic studies. Diagn Microbiol Infect Dis 12:421–428
Xiao S, Zhuo C, Zhuo C (2021) In vitro activity of various sulbactam compounds and piperacillin/tazobactam against clinical isolates of different gram-negative bacteria. Comput Math Methods Med ; 2021: 1175379
Wang Q, Wang Z, Zhang F et al (2020) Long-term continuous antimicrobial resistance surveillance among nosocomial gram-negative bacilli in China from 2010 to 2018 (CMSS). Infect Drug Resist 13:2617–2629
Meatherall BL, Gregson D, Ross T et al (2009) Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med 122:866–873
Chetcuti Zammit S, Azzopardi N, Sant J (2014) Mortality risk score for Klebsiella pneumoniae bacteraemia. Eur J Intern Med 25:571–576
Acknowledgements
This work was supported by the Tri-Service General Hospital under grant numbers TSGH-E-112286, TSGH-E-112253 and TSGH-E-113284; National Defense Medical Center under grant numbers MND-MAB-D-112115, MND-MAB-D-113078, CMNDMC11206 and CMNDMC11303; National Science and Technology Council under grant numbers NSTC 112-2314-B-016-023 and 112-2314-B-016-039; and TTY Biopharma Company. The latter had no role in the study design, data collection and analysis, article preparation, or the decision to submit.
Funding
This work was supported by the Tri-Service General Hospital under grant numbers TSGH-E-112286, TSGH-E-112253 and TSGH-E-113284; National Defense Medical Center under grant numbers MND-MAB-D-112115, MND-MAB-D-113078, CMNDMC11206 and CMNDMC11303; National Science and Technology Council under grant numbers NSTC 112-2314-B-016-023 and 112-2314-B-016-039; and TTY Biopharma Company. The latter had no role in the study design, data collection and analysis, article preparation, or the decision to submit.
Author information
Authors and Affiliations
Contributions
Study design: Tsung-Ta Chiang, Shang-Yi Lin, Po-Liang Lu, Ting-Shu Wu, Shian-Sen Shie, Feng-Yee Chang, Ya-Sung Yang, Mao-Wang Ho, Chia-Hui Chou, Jien-Wei Liu, Zhi-Yuan Shi, Yin-Ching Chuang, Hung-Jen Tang, Fu-Der Wang; Data collection: Tsung-Ta Chiang, Shang-Yi Lin, Po-Liang Lu, Ting-Shu Wu, Shian-Sen Shie, Feng-Yee Chang, Ya-Sung Yang, Mao-Wang Ho, Chia-Hui Chou, Jien-Wei Liu, Zhi-Yuan Shi, Yin-Ching Chuang, Hung-Jen Tang, Fu-Der Wang; Data analysis: Tsung-Ta Chiang, Ming-Hsien Chiang, Shang-Yi Lin, Po-Liang Lu, Ting-Shu Wu, Shian-Sen Shie, Feng-Yee Chang, Ya-Sung Yang, Mao-Wang Ho, Chia-Hui Chou, Jien-Wei Liu, Zhi-Yuan Shi, Yin-Ching Chuang, Hung-Jen Tang, Fu-Der Wang; Statistical analysis: Tsung-Ta Chiang, Ming-Hsien Chiang, Ya-Sung Yang; Manuscript preparation: Tsung-Ta Chiang, Ming-Hsien Chiang, Feng-Yee Chang, Ya-Sung Yang, Fu-Der Wang; Review and approval: all authors.
Corresponding authors
Ethics declarations
Ethical approval
The study was approved by the Institutional Review Board of the Tri-Service General Hospital (TSGH IRB No. 1-106-05-116) and the institutional review boards of all other participating hospitals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiang, TT., Chiang, MH., Tang, HJ. et al. Multicenter study on clinical outcomes and poor prognostic factors in patients with Klebsiella pneumoniae bacteremia receiving cefoperazone/sulbactam treatment. Eur J Clin Microbiol Infect Dis (2024). https://doi.org/10.1007/s10096-024-04892-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10096-024-04892-x