Abstract
Introduction
Ceftazidime/avibactam-resistance in Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) is a topic of great interest for epidemiological, diagnostic, and therapeutical reasons. However, data on its prevalence and burden on mortality in patients with bloodstream infection (BSI) are lacking. This study was aimed at identifying risk factors for mortality in patients suffering from ceftazidime/avibactam-resistant KPC-Kp BSI.
Methods
An observational retrospective study (January 2018–December 2022) was conducted at a tertiary hospital including all consecutive hospitalized adult patients with a ceftazidime/avibactam-resistant KPC-Kp BSI. Data on baseline clinical features, management, and admission outcomes were analyzed.
Results
Over the study period, among all the KPC-Kp BSI events recorded, 38 (10.5%) were caused by ceftazidime/avibactam-resistant KPC-Kp strains, 37 events being finally included. The ceftazidime/avibactam-resistant KPC-Kp strains revealed susceptibility restoration to at least one carbapenem in more than 60% of cases. In-hospital and 30-day all-cause mortality rates were 22% and 16.2%, respectively. Non-survivors suffered from more baseline comorbidities and experienced a more severe ceftazidime/avibactam-resistant KPC-Kp BSI presentation (i.e., both the Pitt Bacteremia and INCREMENT-CPE scores were significantly higher). Presenting with a higher Charlson Comorbidity Index, chronic kidney disease—KDIGO stage 3A or worse—having recently gone through renal replacement therapy, having suffered from an acute kidney injury following the ceftazidime/avibactam-resistant KPC-Kp BSI, and being admitted for cardiac surgery were the strongest predictors of mortality.
Conclusion
Ceftazidime/avibactam resistance in KPC-Kp BSI easily emerged in our highly KPC-Kp endemic area with remarkable mortality rates. Our findings might provide physicians possibly actionable information when managing patients with a ceftazidime/avibactam-resistant KPC-Kp BSI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) emerged globally as one of the most clinically relevant pathogen in view of its wide dissemination in healthcare facilities, the limited number of effective treatment options, and the resulting high mortality rates of the associated infections [1]. The alert issued in Italy based on data from 2014–2017 highlighted the endemic burden of KPC-Kp often characterized by a relevant number of bloodstream infections (BSIs) and endangering patient safety [2]. In 2018, the novel β-lactam/β-lactamase inhibitor combination ceftazidime/avibactam was introduced into clinical practice having shown excellent clinical activity against KPC-Kp [3,4,5,6,7,8,9]. More recently, it has been reported as the only independent survival predictor among KPC-Kp BSI patients [6, 9]. However, 5 years following the onset of clinical use, in vivo selection events of ceftazidime/avibactam-resistant strains [10, 11] and nosocomial outbreaks of ceftazidime/avibactam-resistant KPC-Kp have been reported [12,13,14]. Acquired resistance to ceftazidime/avibactam seems to be mainly due to amino acid substitutions in β-lactamases, alterations of ompK35/36 porins, and/or overexpression of efflux pumps. At the present time, from an epidemiological point of view, the most common resistance mechanism is the expression of KPC variants consisting of single amino acid substitutions between the positions 164–179 in the Ω loop region (most notably the Asp179Tyr—D179Y substitution). These variants are characterized by the loss of carbapenemase activity and the restoration of susceptibility to carbapenems, together with a concomitant diminished binding to avibactam. Other KPC variants encode mutations outside the Ω loop region, not necessarily having established a relationship with a previous ceftazidime/avibactam treatment [15,16,17,18,19]. Notably, while molecular testing is capable of detecting all KPC mutants, phenotypic and immunochromatographic methods revealed significant issues regarding the detection of KPC variants with diminished carbapenemase activity [19,20,21,22]. Moreover, strains harboring specific KPC mutants have been shown to have co-resistance towards both ceftazidime/avibactam and the recently approved cefiderocol [23, 24], meropenem-vaborbactam, and imipenem-relebactam [25]. Altogether, the spread of the KPC enzyme bearing high evolutionary potential, the limitations of diagnostic tests resulting in missed infection control practices, and the acquisition of resistance to newly introduced drugs might be paving the way to widespread waves of antimicrobial resistance. Ceftazidime/avibactam-resistant KPC-Kp strains are a topic of great interest and have been well described from an evolutionary point of view [26, 27], but data on their prevalence in KPC-Kp BSI and burden on mortality are lacking. We have sought to retrospectively pinpoint clinical features of relevance for mortality in patients with ceftazidime/avibactam-resistant KPC-Kp BSI in a high KPC-Kp endemicity setting over a 5-year period starting from the introduction of ceftazidime/avibactam into clinical practice. Accordingly, the present study was aimed at identifying risk factors for in-hospital and 30-day all-cause mortality to provide microbiologists and physicians with prognostic and possibly actionable information when managing patients with a ceftazidime/avibactam-resistant KPC-Kp BSI.
Material and methods
Study design
This observational retrospective study was conducted from January 1 2018 to December 31 2022 in Azienda Ospedaliero-Universitaria “Città della Salute e della Scienza di Torino” (Turin, Italy), one of the largest health center in Europe with a total bed capacity of 1900. All consecutive adult patients hospitalized due to ceftazidime/avibactam-resistant KPC-Kp BSI were included. Patient electronic medical records were used for collecting the following data: patient baseline clinical characteristics including Charlson Comorbidity Index, reason for admission, source of the ceftazidime/avibactam-resistant KPC-Kp BSI, ceftazidime/avibactam-resistant KPC-Kp BSI severity including Pitt Bacteremia score and INCREMENT-CPE score, ceftazidime/avibactam-resistant KPC-Kp antimicrobial susceptibility testing results, ceftazidime/avibactam-resistant KPC-Kp BSI target antibiotic therapy, and admission outcomes/complications.
Definitions
Blood culture (BC) positivity for a ceftazidime/avibactam-resistant KPC-Kp strain and concomitant systemic inflammatory response syndrome signs were the criteria used to define a ceftazidime/avibactam-resistant KPC-Kp BSI episode. The ceftazidime/avibactam-resistant KPC-Kp BSI onset was defined as the date of the index positive BC was performed. The National Healthcare Safety Network criteria were used to define the source of the ceftazidime/avibactam-resistant KPC-Kp BSI, primary ceftazidime/avibactam-resistant KPC-Kp BSI being defined as not secondary to infection at another body site [28]. Active antibiotic therapy was defined when started within 48 h of the ceftazidime/avibactam-resistant KPC-Kp BSI onset and when the ceftazidime/avibactam-resistant KPC-Kp strain was susceptible in vitro to at least one prescribed drug. Antibiotic combination therapy included at least one other antimicrobial administered for ≥ 48 h. Prolonged infusion of antibiotic was defined as an antibiotic dose administered over a period of at least 2 h. Antibiotic dose adjusted for impaired renal function was defined as EUCAST dose [29] reduction of renally cleared antibiotics in patients with impaired renal function. Superimposed and/or following the ceftazidime/avibactam-resistant KPC-Kp BSI treatment candidemia was defined as a BSI event documented by BC positivity for a Candida spp. strain during or following the ceftazidime/avibactam-resistant KPC-Kp BSI treatment. KPC-Kp infection relapse was defined as the onset of a second microbiologically documented KPC-Kp infection in a patient whose original ceftazidime/avibactam-resistant KPC-Kp BSI had been clinically cured with resolution of symptoms/signs and negative BCs. Ceftazidime/avibactam exposure was defined as documented treatment with ceftazidime/avibactam for more than 72 h in 90 days preceding the ceftazidime/avibactam-resistant KPC-Kp BSI.
Microbiological diagnostics
The BACT/ALERT Virtuo (bioMérieux, Marcy l’Ètoile, France) and BACT/ALERT FA and FN Plus BCs bottles (bioMérieux, Marcy l’Ètoile, France) were the BCs detection system and bottles used during this study, respectively. Positive BCs were subjected to Gram staining and subculture on appropriate solid medium. MALDI-TOF analysis (Bruker DALTONIK GmbH, Bremen, Germany) and Xpert Carba-R on GeneXpert platform (Cepheid, Sunnyvale, CA) were used for species identification and detection of KPC production in K. pneumoniae isolates, respectively. Susceptibility to major antimicrobials was performed using a commercial microdilution system (Panel NMDR, MicroScan WalkAway 96 Plus, Beckman Coulter, Switzerland), whereas that to ceftazidime/avibactam and meropenem/vaborbactam by the E-test method (BioMérieux, Marcy l’Ètoile, France). Antimicrobial susceptibilities were interpreted according to the current EUCAST clinical breakpoints, with the ceftazidime/avibactam resistance clinical breakpoint being set at > 8 mg/L [29].
Patients underwent screening rectal swab according to the program of surveillance and control of healthcare-associated multidrug resistant Gram-negative infections of our institution that requires rectal screening swabs for new admissions and for inpatients on a weekly basis. The automated direct plating Wasp® instrument (Copan, Brescia, Italy) was used to inoculate the FecalSwab™ system (Copan, Brescia, Italy) on Brilliance CRE medium (Oxoid Ltd, Hampshire, UK).
Statistical analysis
Summary descriptive statistics were presented as absolute counts (n) and percentage for categorical data and as median and interquartile range (IQR) for continuous variables. The Fisher’s exact test was used to compare proportions in the case of categorical variables between patients dying in-hospital and/or at 30 days following admission and those surviving, independently (i.e., 2 × 2 matrixes). In turn, for continuous features, the Mann–Whitney U test was employed. Exact p-values were reported in both cases. With the goal of dissecting the relative contribution of each variable for mortality while adjusting for every other feature (Ceteris paribus) and reducing the output variance, a random forest (RF) classifier was employed (1000 trees, 2/3rd of the observations sampled at each iteration, seven variables randomly sampled at each split with replacement). Variable importance was ranked based on the GINI coefficient (average total decrease in node impurity), for in-hospital and 30-day all-cause mortality separately. For each individual patient, the model’s prediction was decomposed based on the contribution of the individual explanatory variables for that unique instance (all else held constant) and summarized in breakdown plots. For further scrutiny, this multivariate analysis was “optimized” using a forward stepping feature selection, settling on the simplest model among those with lower out-of-bag error rates. We allowed the process to converge on the same set of features for in-hospital and 30-day all-cause mortality, which consisted of kidney disease history (chronic and acute kidney injury following the ceftazidime/avibactam-resistant KPC-Kp BSI), recent renal replacement therapy history, the absolute Charlson Comorbidity index score, and whether cardiac surgery had been the reason for admission (Supplementary Figure S1). Features pertaining to hospitalization time/time from KPC-Kp BSI onset to discharge or death were deliberately left out of the model as these were not baseline predictors (i.e., could only be known after the fact) and were thus of questionable clinical relevance for future cases. Data analysis was performed in R version 4.2.2.
Results
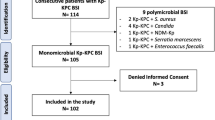
Over the study period, 362 KPC-Kp BSI episodes have been recorded. Among these, 38 (10.5%) were caused by ceftazidime/avibactam-resistant KPC-Kp strains and 37 events (from 37 individual patients) were included in this study (Supplementary Figure S2). Clinical features of patients included in the study are reported in Table 1. The median age was 59 years [IQR 52–69], 68% (n = 25) were men, 92% (n = 34) had been critically ill patients during the 30 days preceding the ceftazidime/avibactam-resistant KPC-Kp BSI onset, and 49% (n = 18) had been treated with ceftazidime/avibactam in the 90 days preceding the ceftazidime/avibactam-resistant KPC-Kp BSI. The median Charlson Comorbidity Index was 3 [IQR 1–5], and the comorbidities mainly observed were cardiovascular disease (35%, n = 13), diabetes (24%, n = 9), chronic respiratory disease (24%, n = 9), chronic kidney disease (Kidney Disease, Improving Global Outcomes 2012, stage 3A or worse, 24%, n = 9), and obesity (24%), with 24% (n = 9) of patients being solid-organ transplant recipients. Twenty-seven percent of patients underwent renal replacement therapy during the 30 days preceding the ceftazidime/avibactam-resistant KPC-Kp BSI onset. Eighty-nine percent of patients were KPC-Kp rectal carriers at the time of the ceftazidime/avibactam-resistant KPC-Kp BSI onset and in 12.1% (n = 4) of these a ceftazidime/avibactam-resistant KPC-Kp strain detected. The median total length-of-stay, length-of-stay before the ceftazidime/avibactam-resistant KPC-Kp BSI onset, and time from the ceftazidime/avibactam-resistant KPC-Kp BSI onset to discharge or death were 95 [IQR 52–150], 35 [IQR 20–72], and 44 [IQR 20–84] days, respectively. The main reasons for admission were sepsis (29.7%, n = 11), cardiac surgery (22%, n = 8), and other surgery (24%, n = 9). The main source of ceftazidime/avibactam-resistant KPC-Kp BSI was the respiratory tract (38%, n = 14), as 41% (n = 15) of cases were not secondary to infection from another site. The median Pitt Bacteremia and INCREMENT-CPE scores were 3 [IQR 1–5] and 6 [IQR 5–11], respectively. Ceftazidime/avibactam-resistant KPC-Kp strains included in the study were susceptible to meropenem, imipenem, gentamicin, and colistin in 62% (n = 23), 57% (n = 21), 51% (n = 19), and 46% (n = 17) of cases, respectively. Meropenem/vaborbactam showed higher activity (93%), but this activity was assessed on 14 strains only. Patients were mainly treated with combination of two antimicrobials (51%, n = 19), and 59% (n = 22) received active antibiotic therapy within 48 h of the ceftazidime/avibactam-resistant KPC-Kp BSI onset. Aminoglycosides- and meropenem/vaborbactam-including regimens were the treatment options mainly prescribed. Prolonged infusion of antibiotic ≥ 2 h and antibiotic dose adjusted for impaired renal function were carried out in 67.6% (n = 25) and 18.9% (n = 7) of cases, respectively. KPC-Kp infection relapse occurred in 18.9% (n = 7) of patients. In-hospital and 30-day all-cause mortality rates were 22% (n = 8) and 16.2% (n = 6), respectively.
Patients who did not survive the hospital admission (Table 2) had significantly higher Charlson Comorbidity Indexes (p-value = 0.04), were more likely to suffer from chronic kidney disease (p-value = 0.01), had more often gone through renal replacement therapy during the 30 days preceding the ceftazidime/avibactam-resistant KPC-Kp BSI onset (p-value < 0.01), experienced a shorter time from the ceftazidime/avibactam-resistant KPC-Kp BSI onset to discharge or death (p-value = 0.02), were more likely to be admitted due to cardiac surgery (p-value < 0.01), and suffered from a more severe ceftazidime/avibactam-resistant KPC-Kp BSI (higher Pitt Bacteremia score, p-value 0.02; higher INCREMENT-CPE score, p-value = 0.03; more often developed shock and acute kidney injury, p-value = 0.03 and p-value < 0.01, respectively). Similarly, patients who did not survive past 30 days after the BSI (Table 2) had a significantly higher Charlson Comorbidity Index (p-value = 0.02), more often suffered from chronic kidney disease (p-value < 0.01), have more often gone through renal replacement therapy during the 30 days preceding the ceftazidime/avibactam-resistant KPC-Kp BSI onset (p-value < 0.01), experienced a shorter time from the ceftazidime/avibactam-resistant KPC-Kp BSI onset to discharge or death (p-value = 0.01), and suffered from a more severe ceftazidime/avibactam-resistant KPC-Kp BSI (more patients with INCREMENT-CPE score ≥ 8, p-value = 0.02; shock, p-value = 0.02; and acute kidney injury, p-value < 0.01). No statistically significant differences between survivors and non-survivors were noted for the KPC-Kp susceptibility pattern, KPC-Kp BSI antibiotic management, and complications following ceftazidime/avibactam-resistant KPC-Kp BSI. The overall distribution for the reported clinical scores is in Supplementary Figure S3. From all the features under consideration, the ensemble model employed to parse the true predictors from the data has consistently shown across both its simplest (data not shown) and most comprehensive (Fig. 1) versions that the presence of chronic kidney disease, acute kidney injury following the ceftazidime/avibactam-resistant KPC-Kp BSI, having recently gone through renal replacement therapy, having a higher Charlson Comorbidity Index, and being admitted for cardiac surgery were the driving features when it comes to accurately predicting whether a patient was likely to die due to ceftazidime/avibactam-resistant KPC-Kp BSI. Accordingly, these features were all positively associated with a higher chance of dying (both in-hospital and 30 days thereafter), while lower comorbidity scores, longer admission periods, and the absence of kidney injury/disease/cardiac surgery/renal replacement therapy have consistently reduced the likelihood of dying (Fig. 2).
Variable importance (weighted average mean decrease Gini / node impurity) for the random forest classifiers in explanatory mode including every single variable under analysis for in-hospital (left) and 30-day all-cause mortality (right) among patients with ceftazidime/avibactam resistant KPC-producing Klebsiella pneumoniae bloodstream infection
Breakdown plots with the contribution of each individual explanatory variable at the individual patient level. Two representative examples of both positive and negative cases for in-hospital and 30-day all-cause mortality in ceftazidime/avibactam-resistant KPC-producing Klebsiella pneumoniae bloodstream infections. Absolute values for continuous features and 0/1 (no/yes) for categorial ones. The intercept term represents the average predicted probability of death across the entire cohort, and subsequent entries display how that prediction changes based on the observed value of each explanatory variable (fixing the effect of every other variable). An individual feature contribution is influenced not only by its overall importance across the entire cohort but also by how much of an effect that variable had in explaining that specific patient’s outcome. Explanatory analysis, not intended for prediction/classification purposes
Discussion
This work represents the largest study published to date on factors associated with mortality in patients hospitalized due to ceftazidime/avibactam-resistant KPC-Kp BSI, shedding some light over the associated mortality patterns 5 years after the introduction of this antibiotic into clinical practice which has nonetheless improved clinical outcomes in KPC-Kp infections [2,3,4,5,6,7,8]. Ceftazidime/avibactam resistance in KPC-Kp BSI effortlessly emerged in our highly KPC-Kp endemic area. The ceftazidime/avibactam-resistant isolates revealed susceptibility restoration to at least one carbapenem in more than 60% of cases. The overall mortality across the entire cohort was remarkable for both in-hospital and 30-day thereafter. Perhaps not surprising, patients who ended up dying suffered from more baseline comorbidities and experienced a more severe ceftazidime/avibactam-resistant KPC-Kp BSI presentation (i.e., both the Pitt Bacteremia and INCREMENT-CPE scores were significantly higher). Among such comorbidities, kidney disease (chronic—KDIGO stage 3A or worse—and acute kidney injury following the ceftazidime/avibactam-resistant KPC-Kp BSI onset) stood out and one of the strongest predictive factors. In parallel, renal replacement therapy was also much more often observed in patients failing to survive the ceftazidime/avibactam-resistant KPC-Kp BSI. Notably, of all the reasons for admission, cardiac surgery was the one most strongly associated with KPC-Kp BSI mortality. In contrast, the KPC-Kp susceptibility pattern, history of previous ceftazidime/avibactam treatment, the onset of concomitant and/or superimposed candidemia, and developing a KPC-Kp infection relapse presented a non-negligible yet much more modest role in predicting patient mortality.
In the light of the excellent clinical results obtained [3,4,5,6,7,8,9], the judicious prescription of ceftazidime/avibactam and the implementation of a surveillance system dedicated to resistance emergence detection should be aimed at. For the time being, KPC-Kp resistance to ceftazidime/avibactam is not perceived as a problem at scale, being deemed a rather uncommon phenomenon [11]. Accordingly, during the first wave of the SARS-CoV-2 pandemic, a Southern Italian multicenter surveillance study reported a single case of ceftazidime/avibactam-resistant KPC-Kp throughout an entire 6-month carbapenem-non-susceptible Enterobacterales isolates collection period [30]. In contrast, although an outbreak occurred in a COVID-19 ICU [12], the prevalence of ceftazidime/avibactam resistance in KPC-Kp BSI isolates in our center has remained stable at around 10% [31] reaching values more similar to those published by Gaibani et al. for the period 2018–2020 in another Northern Italian center [32].
The susceptibility patterns of ceftazidime/avibactam-resistant KPC-Kp strains herein reported are in line with other reports [11], whereby a susceptibility restoration phenomenon to at least one carbapenem (despite no association with previous ceftazidime/avibactam exposure) was observed. The fact that ceftazidime/avibactam-resistant KPC-Kp isolates with reduced carbapenemase activity are the most widespread is not inconsequential. From a diagnostic standpoint, these may often go undetected, especially by the phenotypic methods most commonly used in hospital surveillance systems [19,20,21,22]. From a therapeutic perspective, the effectiveness of pre-emptive therapy with ceftazidime/avibactam might be limited when ceftazidime/avibactam-resistant KPC variants start to circulate in a hospital, even considering that ceftazidime/avibactam exposure induces resistance development more frequently in the blood and respiratory tract than in the rectum [33], highlighting the need to update the diagnostic protocols of surveillance cultures [12].
Mortality rates of patients with ceftazidime/avibactam-resistant KPC-Kp infections have been estimated in a review paper by Di Bella et al. at around 37% [11], moving us backwards all the way to when ceftazidime/avibactam was not yet available as therapeutic option. In the present study, both in-hospital and 30-day all-cause mortality rates were lower than the aforementioned, being closer to those reported among patients suffering from ceftazidime/avibactam-susceptible KPC-Kp infections and treated with it [2, 6].
Notably, even though most of the ceftazidime/avibactam-resistant KPC-Kp strains isolated for the purposes of this study had restored susceptibility to carbapenems and most patients had been treated within 48 h of the BSI onset, no single therapeutic regimen could be pinpointed as factor associated with reduced mortality in this situation. In all likelihood, data is too sparse in the present study in order to confidently dissect these so that whether such mortality-reducing therapeutic regimens exist remains an open question.
The present study is limited in its retrospective nature, because it has been conducted at a single center, and due to the limited number of cases for each combination of features of interest. Nonetheless, this study emphasizes the importance of epidemiological surveillance with the goal of keeping resistance to ceftazidime/avibactam at bay and highlights the clinical features that might be relevant for mortality. Whether these and other features are actionable remains to be seen, especially since all the important predictors herein highlighted are not modifiable.
Current efforts should be focused on the early identification of ceftazidime/avibactam-resistant KPC-Kp and respective susceptibility patterns to avoid rounds of failed treatment attempts, on good coordination and implementation of a quick and efficient surveillance system. The identification of how best to proceed at the individual patient level in the face of a ceftazidime/avibactam-resistant KPC-Kp BSI as identified may prove useful for individual patient risk stratification.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL (2012) Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. https://doi.org/10.1128/CMR.05035-11
Iacchini S, Sabbatucci M, Gagliotti C, Rossolini GM, Moro ML, Iannazzo S et al (2019) Bloodstream infections due to carbapenemase-producing Enterobacteriaceae in Italy: results from nationwide surveillance, 2014 to 2017. Euro Surveill 24:1800159. https://doi.org/10.2807/1560-7917.ES.2019.24.5.1800159
Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV et al (2017) Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-e917. https://doi.org/10.1128/AAC.00883-17
van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F et al (2018) Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 66:163–171. https://doi.org/10.1093/cid/cix783
Tumbarello M, Raffaelli F, Giannella M, Mantengoli E, Mularoni A, Venditti M et al (2021) Ceftazidime-avibactam use for Klebsiella pneumoniae carbapenemase-producing K. pneumoniae infections: a retrospective observational multicenter study. Clin Infect Dis 73:1664–76. https://doi.org/10.1093/cid/ciab176
Karaiskos I, Daikos GL, Gkoufa A, Adamis G, Stefos A, Symbardi S et al (2021) Ceftazidime/avibactam in the era of carbapenemase-producing Klebsiella pneumoniae: experience from a national registry study. J Antimicrob Chemother 76:775–783. https://doi.org/10.1093/jac/dkaa503
Castón JJ, Cano A, Pérez-Camacho I, Aguado JM, Carratalá J, Ramasco F et al (2022) Impact of ceftazidime/avibactam versus best available therapy on mortality from infections caused by carbapenemase-producing Enterobacterales (CAVICOR study). J Antimicrob Chemother 77:1452–1460. https://doi.org/10.1093/jac/dkac049
Satlin MJ, Chen L, Gomez-Simmonds A, Marino J, Weston G, Bhowmick T et al (2022) Impact of a rapid molecular test for Klebsiella pneumoniae carbapenemase and ceftazidime-avibactam use on outcomes after bacteremia caused by carbapenem-resistant Enterobacterales. Clin Infect Dis 75:2066–2075. https://doi.org/10.1093/cid/ciac354
Boattini M, Bianco G, Charrier L, Comini S, Iannaccone M, Almeida A et al (2023) Rapid diagnostics and ceftazidime/avibactam for KPC-producing Klebsiella pneumoniae bloodstream infections: impact on mortality and role of combination therapy. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-023-04577-x
van Asten SAV, Boattini M, Kraakman MEM, Bianco G, Iannaccone M, Costa C et al (2021) Ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in KPC-producing Klebsiella pneumoniae infections: a case series. J Infect Chemother 27:778–780. https://doi.org/10.1016/j.jiac.2021.01.014
Di Bella S, Giacobbe DR, Maraolo AE, Viaggi V, Luzzati R, Bassetti M et al (2021) Resistance to ceftazidime/avibactam in infections and colonisations by KPC-producing Enterobacterales: a systematic review of observational clinical studies. J Glob Antimicrob Resist 25:268–281. https://doi.org/10.1016/j.jgar.2021.04.001
Bianco G, Boattini M, Bondi A, Comini S, Zaccaria T, Cavallo R et al (2022) Outbreak of ceftazidime-avibactam resistant Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in a COVID-19 intensive care unit, Italy: urgent need for updated diagnostic protocols of surveillance cultures. J Hosp Infect 122:217–219. https://doi.org/10.1016/j.jhin.2022.02.001
Di Pilato V, Principe L, Andriani L, Aiezza N, Coppi M, Ricci S et al (2023) Deciphering variable resistance to novel carbapenem-based β-lactamase inhibitor combinations in a multi-clonal outbreak caused by Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae resistant to ceftazidime/avibactam. Clin Microbiol Infect 29:537.e1-537.e8. https://doi.org/10.1016/j.cmi.2022.11.011
Nicola F, Cejas D, González-Espinosa F, Relloso S, Herrera F, Bonvehí P et al (2022) Outbreak of Klebsiella pneumoniae ST11 resistant to ceftazidime-avibactam producing KPC-31 and the novel variant KPC-115 during COVID-19 pandemic in Argentina. Microbiol Spectr 10:e0373322. https://doi.org/10.1128/spectrum.03733-22
Mueller L, Masseron A, Prod’Hom G, Galperine T, Greub G, Poirel L et al (2019) Phenotypic, biochemical and genetic analysis of KPC-41, a KPC-3 variant conferring resistance to ceftazidime–avibactam and exhibiting reduced carbapenemase activity. Antimicrob Agents Chemother 63:e01111-19. https://doi.org/10.1128/AAC.01111-19
Galani I, Antoniadou A, Karaiskos I, Kontopoulou K, Giamarellou H, Souli M (2019) Genomic characterization of a KPC-23-producing Klebsiella pneumoniae ST258 clinical isolate resistant to ceftazidime–avibactam. Clin Microbiol Infect 25(763):e5-8. https://doi.org/10.1016/j.cmi.2019.03.011
García J, Nastro M, Cejas D, Santana G, Mancino MB, Hidalgo M et al (2020) Emergence of ceftazidime/avibactam resistance in KPC-8-producing Klebsiella pneumoniae in South America. Clin Microbiol Infect 26:1264–1265. https://doi.org/10.1016/j.cmi.2020.03.013
Poirel L, Vuillemin X, Juhas M, Masseron A, Bechtel-Grosch U, Tiziani S et al (2020) KPC-50 confers resistance to ceftazidime–avibactam associated with reduced carbapenemase activity. Antimicrob Agents Chemother 64:e00321-20. https://doi.org/10.1128/AAC.00321-20
Bianco G, Boattini M, Iannaccone M, Cavallo R, Costa C (2020) Bloodstream infection by two subpopulations of Klebsiella pneumoniae ST1685 carrying KPC-33 or KPC-14 following ceftazidime/avibactam treatment: considerations regarding acquired heteroresistance and choice of carbapenemase detection assay. J Antimicrob Chemother 75:3075–3076. https://doi.org/10.1093/jac/dkaa283
Gaibani P, Lombardo D, Foschi C, Re MC, Ambretti S (2020) Evaluation of five carbapenemase detection assays for Enterobacteriaceae harbouring blaKPC variants associated with ceftazidime/avibactam resistance. J Antimicrob Chemother 75:2010–2013. https://doi.org/10.1093/jac/dkaa079
Bianco G, Boattini M, Iannaccone M, Bondi A, Ghibaudo D, Zanotto E et al (2021) Carbapenemase detection testing in the era of ceftazidime/avibactam-resistant KPC-producing Enterobacterales: a 2-year experience. J Glob Antimicrob Resist 24:411–414. https://doi.org/10.1016/j.jgar.2021.02.008
Hernández-García M, Castillo-Polo JA, Cordero DG, Pérez-Viso B, García-Castillo M, Saez de la Fuente J, Morosini MI et al (2022) Impact of ceftazidime-avibactam treatment in the emergence of novel KPC variants in the ST307-Klebsiella pneumoniae high-risk clone and consequences for their routine detection. J Clin Microbiol 60:e0224521. https://doi.org/10.1128/jcm.02245-21
Bianco G, Boattini M, Comini S, Iannaccone M, Bondi A, Cavallo R et al (2022) In vitro activity of cefiderocol against ceftazidime-avibactam susceptible and resistant KPC-producing Enterobacterales: cross-resistance and synergistic effects. Eur J Clin Microbiol Infect Dis 41:63–70. https://doi.org/10.1007/s10096-021-04341-z
Poirel L, Sadek M, Kusaksizoglu A, Nordmann P (2022) Co-resistance to ceftazidime-avibactam and cefiderocol in clinical isolates producing KPC variants. Eur J Clin Microbiol Infect Dis 41:677–680. https://doi.org/10.1007/s10096-021-04397-x
Gaibani P, Bianco G, Amadesi S, Boattini M, Ambretti S, Costa C (2022) Increased blaKPC copy number and OmpK35 and OmpK36 porins disruption mediated resistance to imipenem/relebactam and meropenem/vaborbactam in a kpc-producing Klebsiella pneumoniae clinical isolate. Antimicrob Agents Chemother 66:e0019122. https://doi.org/10.1128/aac.00191-22
Hobson CA, Pierrat G, Tenaillon O, Bonacorsi S, Bercot B, Jaouen E et al (2022) Klebsiella pneumoniae carbapenemase variants resistant to ceftazidime-avibactam: an evolutionary overview. Antimicrob Agents Chemother 66:e0044722. https://doi.org/10.1128/aac.00447-22
Carattoli A, Arcari G, Bibbolino G, Sacco F, Tomolillo D, Di Lella FM et al (2021) Evolutionary trajectories toward ceftazidime-avibactam resistance in Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother 65:e0057421. https://doi.org/10.1128/AAC.00574-21
CDC/NHSN Surveillance Definitions for Specific Types of Infections. Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf. Accessed: 31 October 2022
https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf. Accessed: 31 October 2022
La Bella G, Lopizzo T, Lupo L, Angarano R, Curci A, Manti B et al (2022) In vitro activity of ceftazidime/avibactam against carbapenem-nonsusceptible Klebsiella penumoniae isolates collected during the first wave of the SARS-CoV-2 pandemic: a Southern Italy, multicenter, surveillance study. J Glob Antimicrob Resist 31:236–238. https://doi.org/10.1016/j.jgar.2022.09.013
Bianco G, Boattini M, Comini S, Iannaccone M, Casale R, Allizond V et al (2022) Activity of ceftolozane-tazobactam, ceftazidime-avibactam, meropenem-vaborbactam, cefiderocol and comparators against Gram-negative organisms causing bloodstream infections in Northern Italy (2019–2021): emergence of complex resistance phenotypes. J Chemother 34:302–310. https://doi.org/10.1080/1120009X.2022.2031471
Gaibani P, Lombardo D, Bussini L, Bovo F, Munari B, Giannella M et al (2021) Epidemiology of meropenem/vaborbactam resistance in KPC-producing Klebsiella pneumoniae causing bloodstream infections in Northern Italy, 2018. Antibiotics 10:536. https://doi.org/10.3390/antibiotics10050536
Gaibani P, Bovo F, Bussini L, Bartoletti M, Lazzarotto T, Viale P et al (2023) Colonization by ceftazidime/avibactam-resistant KPC-producing Klebsiella pneumoniae following therapy in critically ill patients. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2023.01.012
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. This research was supported by EU funding within the MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
Author information
Authors and Affiliations
Contributions
BM and BG designed the study; BM, BG, CS, and CS acquired data; BM and BP analyzed and interpreted data; BM wrote the paper; AA, CC, DRFG, and CR supervised the study; all authors revised the article critically and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki. Formal ethical approval was obtained by our center’s institutional review board (Protocol No. 0048443).
Informed consent statement
Informed consent was waived due to the retrospective nature of the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boattini, M., Bianco, G., Bastos, P. et al. Prevalence and mortality of ceftazidime/avibactam-resistant KPC-producing Klebsiella pneumoniae bloodstream infections (2018–2022). Eur J Clin Microbiol Infect Dis 43, 155–166 (2024). https://doi.org/10.1007/s10096-023-04712-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04712-8