Abstract
Introduction
In the present study, we aimed to describe the proportion of carbapenem-resistant Klebsiella pneumoniae bloodstream infection (CRKP-BSI) in KP-BSI in one Chinese tertiary hospital over 10 years and determine the risk factors and outcomes of CRKP-BSI.
Methods
We retrospectively analyzed clinical and microbiological data of patients with KP-BSI from January 2010 to December 2019 to identify risk factors, clinical features, and outcomes using multivariate logistic regression analysis. KP-BSI only included monomicrobial BSI and health care-acquired BSI.
Results
Among the total 687 isolates of KP-BSI in this study, the rate of CRKP was 39.0% (268/687); this rate in the intensive care unit (ICU) was 65.6% and that in seven high-risk departments (including four ICUs, respiratory medicine, gastroenterology medicine, and hepatobiliary surgery) was 74.6%. The annual rate of CRKP in KP-BSI ranged from 0.0% in 2010 to 54.5% in 2019. The 28-day mortality was 36.2% in patients with CRKP-BSI and 11.7% in those with carbapenem-susceptible K. pneumoniae (CSKP) BSI. Multivariable logistic regression analysis showed that prior ICU stay (odds ratio [OR] 2.485, P < 0.001), hospital stay ≥ 30 days prior to BSI (OR 1.815, P = 0.007), prior mechanical ventilation (OR 2.020, P = 0.014), prior urinary catheter (OR 1.999, P = 0.003), prior carbapenem use (OR 3.840, P < 0.001), hepatobiliary disease (OR 2.943, P < 0.001), pancreatitis (OR 2.700, P = 0.026), and respiratory disease (OR 2.493, P = 0.009) were risk factors of CRKP-BSI. Patients with a first admission (OR 0.662, P = 0.046) had a lower percentage of CRKP-BSI.
Conclusion
The rapidly rising rate of CRKP-BSI in KP with high mortality requires increased attention. Exposure to carbapenems, ICU stay, invasive mechanical ventilation or urinary catheter, prolonged hospital stay, hepatobiliary disease, pancreatitis, and respiratory disease were found to be risk factors for CRKP-BSI. Strict control measures should be implemented to prevent the emergence and spread of CRKP, especially in high-risk departments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
We studied the characteristics of carbapenem-resistant Klebsiella pneumoniae bloodstream infection (CRKP-BSI) in all departments of one Chinese tertiary hospital over 10 years. |
What was learned from the study? |
We included 268 patients with CRKP-BSI and 419 patients with carbapenem-susceptible K. pneumoniae (CSKP) BSI in the study. |
We observed that prior ICU stay, hospital stay ≥ 30 days prior to BSI, prior mechanical ventilation, prior urinary catheter, prior carbapenem use, hepatobiliary disease, pancreatitis, and respiratory disease were associated with CRKP-BSI in our analysis. |
The rapidly rising rate of CRKP-BSI in KP (from 0.0% in 2010 to more than 60% in 2019), with high mortality (36.2%), requires increased attention. |
Introduction
Carbapenem-resistant Klebsiella pneumoniae (CRKP) is rapidly spreading across the globe and has attracted much attention. Indeed, CRKP infections with high morbidity and mortality have become an urgent threat, according to the Centers for Disease Control and Prevention (CDC) [1]. Different detection rates of CRKP in KP are reported in different regions, with proportions exceeding 50% in parts of Europe in 2017 [2] and increasing from 14.1% in 2013 to 24.2% during 2020 in China [3].
Identifying patients at risk of infection with CRKP may help in selecting an appropriate empiric treatment if the patient develops a severe clinical picture consistent with acute infection. However, studies [4, 5] on risk factors for CRKP infection have provided inconsistent results because of sample biases and choice of the control group possibly affecting consistency of the results [6, 7]. Additionally, CRKP detection involves the respiratory tract or urinary tract, as well as bloodstream infection (BSI), which includes infection and colonization with CRKP [4,5,6,7]. So more relevant research is needed. But there is still a lack of large sample research in China.
Therefore, in the present study, we aimed to describe the overall detection rate of CRKP-BSI in KP-BSI among all departments of one Chinese tertiary hospital over 10 years and to determine the risk factors and outcomes of CRKP-BSI. Only monomicrobial and health care-acquired KP-BSI were included.
Methods
Participants
The study hospital is a tertiary teaching hospital with 3800 beds in Beijing. The hospital has approximately 100 wards (35–40 beds per ward) and 10 intensive care units (ICUs; 15–20 beds per ICU). Each disease specialty department has approximately 3–5 wards, with some including one ICU. There are approximately 150,000 admissions to the study hospital every year. We retrospectively analyzed patients admitted to the study hospital between January 1, 2010 and December 31, 2019.
Definitions
KP-BSI was diagnosed when at least one blood sample culture was positive for KP and clinical features were compatible with sepsis syndrome. The source of bacteremia was judged retrospectively in accordance with the definitions of the US Centers for Disease Control and Prevention [8]. Hospital-acquired (HA) BSI was defined as the first positive blood culture obtained 48 h or more after hospital admission and with no evidence of infection at admission. If the BSI was directly related to hospitalization or outpatient treatment, it was defined as HA BSI regardless of whether the identification time was 48 h after admission. Community-acquired (CA) BSI was defined as the first positive blood culture obtained less than 48 h after hospital admission. Polymicrobial BSI was defined as two or more clinically important organisms isolated from a single blood culture sample or different blood culture samples within 48 h. Patients with polymicrobial bacteremia or CA infection were excluded. The 28-day mortality was defined as death by any cause within 28 days of BSI onset.
Blood Culture
Blood samples were cultured using the BACT/ALERT® 3D™ system (BD, Sparks, MD, USA) in the microbiology laboratory. Species were identified and then in vitro antibiotic susceptibility was determined with the Vitek II (bioMérieux, France). Lists of antimicrobial categories proposed for antimicrobial susceptibility testing were created using documents and breakpoints from the annually updated Clinical Laboratory Standards Institute (CLSI) [9] from the CLSI 2010 edition to the 2019 edition. CRKP was defined as ertapenem, meropenem, or imipenem resistance.
Data Collection
We collected epidemiological and clinical data from the electronic case collection system [10]; a previous study showed that the sensitivity and specificity of the system in surveillance for HA infections were 98.8% and 93.0%, respectively. The collected data included age, sex, comorbidities, diagnosis on admission, sources of BSI, hospital stay before BSI, invasive devices, carbapenem use for more than 72 h at any point within 30 days prior to BSI, history of surgery, stay in a high-risk department or an ICU, and treatment outcomes. According to the number of cases of CRKP-BSI, the departments involved were divided into three categories: high-risk, low-risk, and risk-free departments. A high-risk department was defined as having more than 10 CRKP-BSI isolates over the 10-year study period. A low-risk department had 1–10 CRKP-BSI isolates over the 10 years. A risk-free department was defined as having no CRKP-BSI isolates over the 10 years.
Ethics
This study was reviewed and approved by the Medical Ethics Committee of PLA General Hospital (Reference no. S2019-142-02), which was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Data for CRKP and carbapenem-susceptible K. pneumoniae (CSKP) were compared using the chi-squared test for equal proportions, with results presented as percentages. Continuous variables were presented as median and interquartile range and were calculated with the Mann–Whitney U test. Categorical variables presented as percentages were compared with the chi-squared test. Multivariable logistic regression models were used to explore independent risk factors for CRKP infection. All variables with a P value < 0.10 in the univariable analysis were included in a multivariable backward logistic regression analysis to assess their relationship with CRKP infection. P < 0.05 was considered to be statistically significant in multivariable logistic regression. The annual change trend in the proportion of CRKP among KP isolates from 2010 to 2019 was analyzed using P for trend. Statistical analysis was performed using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient Clinical Characteristics
We excluded 1148 isolates because of duplicated positive blood cultures (n = 946), CA BSI (n = 129), and polymicrobial bacteremia (n = 73). In total, 687 patients were included in the study. A total of 268 patients with CRKP-BSI and 419 with CSKP-BSI were included. The flowchart of screening, exclusion criteria, and the grouping of study participants is shown in Fig. 1. Table 1 shows the baseline characteristics of patients with KP-BSI. The median age of patients was 60 years, and 72.2% were male patients. There were significant differences in age and sex between the two groups (P < 0.001 vs. P = 0.044). Hepatobiliary disease (n = 222, 32.3%) was the most common diagnosis on admission, followed by other gastrointestinal diseases (n = 71, 10.3%) and hematological disease (n = 71, 10.3%). In total, 65.7% of patients with CRKP and 45.4% of patients with CSKP had one or more comorbidities.
In patients with CRKP-BSI, the most frequent sources of BSI were lung infection (35.4%) and abdominal infection (31.0%). In patients with CSKP-BSI, 64.4% of sources were primary BSI or unknown and 13.4% of sources were abdominal infection. The overall all-cause 7-day, 14-day, and 28-day mortality rates in patients with CRKP-BSI were higher than those of the CSKP group (20.1% vs. 8.1%, 30.0% vs. 9.8%, 36.2% vs. 11.7%; all P < 0.001). The odds ratio (OR) (95% confidence interval) for death owing to CRKP was 3.967 (2.673–5.886).
Prevalence
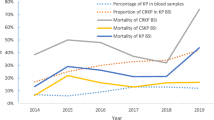
Of the total 687 isolates of KP-BSI, 268 strains (39.0%) were confirmed to be carbapenem resistant. The annual proportion of CRKP among KP isolates ranged from 0.0% in 2010 to 54.5% in 2019 (Fig. 2), which was significant (P for trend < 0.001). The proportion of CRKP in the ICU was 65.6% (143/218), which was significantly higher than that in non-ICU departments at 26.7% (125/469) (χ2 = 94.867, P < 0.001).
Department Distribution
The five highest rates of CRKP in KP were all in the ICU: respiratory ICU (80.0%), neurology ICU (75.0%), surgical ICU (71.8%), emergency ICU (71.0%), and hepatobiliary surgical ICU (70.0%). A total of 165 CRKP-BSI strains were isolated from seven ICUs, accounting for 61.6% of the total cases of CRKP-BSI. ICU departments were not completely consistent with high-risk departments. A total of 198 CRKP-BSI isolates (73.9%) were detected in seven high-risk departments, namely four ICUs and three non-ICU departments (hepatobiliary surgery, respiratory medicine, and gastroenterology medicine), in three ICUs (cardiovascular medicine ICU, cardiovascular surgical ICU, and neurology ICU), and in 18 non-ICU departments belonging to a low-risk department; a total of 70 CRKP-BSI isolates (26.1%) were detected. Risk-free departments included three ICUs (neonatal ICU, neurosurgery ICU, and nephrology ICU) and eight non-ICU departments (Fig. 3).
CRKP-BSI and CSKP-BSI from 2010 to 2019 in different departments. High-risk department: more than 10 CRKP-BSI isolates in 10 years; low-risk department: 1–10 CRKP-BSI isolates in 10 years; risk-free department: 0 CRKP-BSI isolates in 10 years. CSKP carbapenem-susceptible K. pneumoniae, CRKP carbapenem-resistant K. pneumoniae, ICU intensive care unit, BSI bloodstream infection
Secondary Sources of CRKP-BSI
The three specialty departments with the most CRKP-BSIs (surgical ICU, hepatobiliary surgery, and respiratory medicine) were selected to analyze the distribution of secondary sources of BSI (Fig. 4). In 74 patients with CRKP-BSI in hepatobiliary surgery, the leading source was abdominal infection (65.6%), followed by pulmonary infection (17.6%). In 36 patients with CRKP-BSI in respiratory medicine, the leading secondary source of infection was pulmonary infection (69.4%); there was no CRKP-BSI secondary to abdominal infection. There were 56 patients with CRKP-BSI in the surgical ICU. The leading secondary source was pulmonary infection (55.4%), followed by abdominal infection (41.1%).
Composition of likely sources of CRKP-BSI in three high-risk departments. Department of respiratory medicine included four respiratory wards and one respiratory ICU; department of hepatobiliary surgery included five hepatobiliary wards and one ICU. The surgical ICU only included one ICU with 20 beds. CRKP carbapenem-resistant K. pneumoniae, ICU intensive care unit, BSI bloodstream infection
Risk Factors for CRKP Infection
Univariate analysis revealed that age, sex, certain diagnosis on admission (respiratory disease, hematological disease, and pancreatitis), underlying disease, hospital stay, prior ICU stay, use of invasive devices (mechanical ventilation, urinary catheter, central venous catheter), and carbapenem use within 30 days before BSI were positively associated with CRKP infection (P < 0.05); prior surgery, first hospital admission, and certain diagnoses on admission (cardiovascular disease, nervous system disease, other digestive disease, and other disease) were negatively associated with CRKP infection (P > 0.05). In accordance with analysis of the baseline between the two groups, all variables with P < 0.10 were included in the logistic regression model. The results showed that prior ICU stay (OR 2.485, P < 0.001), hospital stay ≥ 30 days prior to BSI (OR 1.815, P = 0.007), prior invasive mechanical ventilation (OR 2.020, P = 0.014), prior invasive urinary catheter (OR 1.999, P = 0.003), carbapenem use within 30 days before BSI (OR 3.840, P < 0.001), hepatobiliary disease (OR 2.943, P < 0.001), pancreatitis (OR 2.700, P = 0.026), and respiratory disease (OR 2.493, P = 0.007) were risk factors for CRKP-BSI. First hospital admission (OR 0.662, P = 0.046) showed lower percentages of CRKP-BSI (Table 2).
Discussion
A number of studies [11, 12] highlighting the emergence of CRKP infection among patients with cancer or in the ICU have been published during the past decade. However, our study involved 268 isolates of HA CRKP-BSI distributed throughout the hospital. In the past 10 years, the proportion of CRKP-BSI has increased rapidly from 0.0% to more than 60%. The 28-day mortality of CRKP-BSI has reached 36.2%, which requires urgent prevention and control measures. In accordance with previous studies [5, 12], our results in logistic regression analysis showed that prior invasive mechanical ventilation or urinary catheter and prior carbapenem use were independent risk factors for CRKP-BSI. To the best of our knowledge, we are the first to report that pancreatitis and hepatobiliary disease were risk factors for CRKP-BSI. The departments in our study hospital with a risk factor of CRKP-BSI are not completely consistent with the ICUs reported in previous studies [12, 13]. Not all ICUs were in high-risk departments. In addition to ICUs, high-risk departments at our study hospital included hepatobiliary surgery, gastroenterology, and respiratory medicine. This suggests that the key departments for CRKP prevention and control in general hospitals should be more accurately determined and it should not be generally assumed that those departments are ICUs.
Our study showed that the overall rate of CRKP in KP infections was 39.0% from 2010 to 2019, but this proportion in the ICUs was 65.6%, which is similar to other reports from China. Li et al. [12] reported that the average CRKP resistance rate was 48.1% among ICUs in six teaching hospitals in Henan Province from 2014 to 2018. The proportion of CRKP-BSI in all of Shanghai Ruijin Hospital and in the ICUs from 2011 to 2015 was 59.62% and 22.18%, respectively [13]. The overall proportions in the aforementioned reports were far beyond the average in the Chinese bacterial surveillance system [3].
A total of 143 isolates of CRKP-BSI were isolated from seven ICUs, accounting for 53.4% of the total CRKP-BSI. However, 200 isolates (74.6%) were detected in seven high-risk departments; four ICUs belonged to high-risk departments, three ICUs to low-risk departments, and three ICUs (nephrology ICU, neurosurgery ICU, and neonatal ICU) belonged to risk-free departments. High-risk departments (e.g., ICUs) have been described as an ideal setting for the creation, dissemination, and amplification of antimicrobial resistance owing to the presence of critically ill patients, the extensive use of invasive procedures, and frequent use of antimicrobials [14].
The overall mortality in our study was 36.2% in patients with CRKP-BSI and 11.7% in patients with CSKP-BSI, which is lower than the rate reported in a meta-analysis; mortality was 54.3% in 722 patients with CRKP-BSI in 20 studies [15]. Tian et al. [13] reported that the 28-day mortality was higher in patients infected with CRKP (33.3%) than in those with CSKP infection (16.0%) (P = 0.04). This may be explained by a relatively different sample population and diverse medical treatment levels. Our study showed that the sources of CRKP-BSI in different departments varied greatly. Respiratory tract infection was the main cause of CRKP-BSI in the respiratory medicine department (accounting for 69.4%), and abdominal infection was the main cause of CRKP-BSI in hepatobiliary surgery (accounting for 65.6%). The difference in infection sources suggests that the environmental source contamination is different (respiratory tract colonization or infection and abdominal colonization or infection mainly contaminates the surrounding environment via sputum and drainage, respectively). Thus, different measures are needed to prevent and control the cross-transmission of respiratory tract and abdominal colonization or infections. The results of our team’s previous research [16] showed that 31.3% of bed units in five ICUs were CRKP-positive on one or more surfaces. Environmental contamination and CRKP-positive patients played an important role in some instances of patient-to-patient transmission in the ICUs investigated. Understanding these factors may help in developing interventions to prevent and control the transmission of CRKP strains.
The identification of different risk factors of CRKP-BSI is an important step to most effectively channel limited resources into prevention and treatment. In accordance with previous studies [5, 12, 13], our results confirmed that prior exposure to carbapenems and prior hospital stay ≥ 30 days were independent risk factors for CRKP-BSI by 3.962 times and 1.795 times. We also found that prior invasive mechanical ventilation and prior urinary catheter were closely related to CRKP-BSI. Among the likely sources of CRKP-BSI, one in three (or 80) were invasive catheter-related (including 52 cases of ventilator-associated pneumonia, 17 of catheter-related BSI, and 11 cases of catheter-associated urinary tract infection). This means that reducing the use of invasive catheters as far as possible could help to prevent CRKP-BSI.
Digestive diseases were first reported as a risk factor for CRKP-BSI in a Chinese study [17] in 2020. In our study, we divided digestive diseases into three subgroups and found that pancreatitis and hepatobiliary diseases were risk factors for CRKP-BSI but other digestive diseases were not; to our knowledge, we are the first to report this. We inferred that KP were normal gastrointestinal tract-colonizing bacteria and that patients with pancreatitis and hepatobiliary diseases had a history of frequent exposure to carbapenems or invasive catheterization.
There are several limitations to this study. First, this was a single-center retrospective study and relied on clinical culture results, which may have introduced bias in the data interpretation. Second, some additional important variables associated with CRKP-BSI could not be included, such as the use of other antibiotics prior to BSI, prior chemotherapy or radiotherapy, and prior CRKP colonization. Additionally, further research is needed to explore the causes of high CRKP infection in pancreatitis or hepatobiliary diseases. Finally, some hospital policy changes that occurred over the 10-year study period may have affected the results. For example, the hospital improved management of the rational use of antibiotics, especially from 2011 to 2014, which effectively reduced antibiotics overuse [18]. Since July 2017, the study hospital has implemented special measures to control carbapenem-resistant bacteria. The results reported by the microbiological team at our study hospital [19, 20] showed that 79.1% (n = 170) of CRKP from 2012 to 2018 in the respiratory department carried the blaKPC-2 gene, and 89.4% of isolates belonged to ST11. KPC-2-producing KP ST11 isolates were dominant in CRKP, which emerged in 2012 and consistently persisted, occasionally causing outbreaks. Further studies are needed for additional drug resistance gene detection and homological identification of the CRKP-BSI group to further explore the sources of and cross-transmission in CRKP.
Conclusions
The rapidly rising incidence of CRKP-BSI, which has high mortality, requires greater attention. A stay in a high-risk department or prolonged hospital stay, prior invasive mechanical ventilation or urinary catheter, and carbapenem use within 30 days before BSI were independent risk factors for CRKP-BSI. Strict control measures should be implemented to prevent the emergence and spread of CRKP, especially in high-risk departments.
References
Patel G, Bonomo RA. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol. 2013;4:48.
European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe - Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017. https://ecdc.europa.eu/sites/portal/files/. Accessed 30 Nov 2018.
CHINET. China antimicrobial resistance surveillance system report. http://www.chinets.com/. Accessed 30 July 2021.
Zhu WM, Yuan Z, Zhou HY. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9:23.
Li J, Li Y, Song N, Chen Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. J Glob Antimicrob Resist. 2020;21:306–13.
Correa L, Martino MDV, Siqueira I, et al. A hospital-based matched case–control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect Dis. 2013;13:80.
Shilo S, Assous MV, Lachish T, et al. Risk factors for bacteriuria with carbapenem-resistant Klebsiella pneumoniae and its impact on mortality: a case-control study. Infection. 2013;41:503–9.
Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40.
CLSI. Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement. M100-S20. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2010.
Du M, Xing Y, Suo J, et al. Real-time automatic hospital-wide surveillance of nosocomial infections and outbreaks in a large Chinese tertiary hospital. BMC Med Inform Decis Mak. 2014;14:9.
Freire MP, Pierrotti LC, Filho HHC, et al. Infections with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in cancer patients. Eur J Clin Microbiol Infect Dis. 2015;34:277–86.
Li Yi, Shen H, Zhu C, Yuetian Yu. Carbapenem-resistant Klebsiella pneumoniae infections among ICU admission patients in central china: prevalence and prediction model. Biomed Res Int. 2019;27(2019):9767313.
Tian L, Tan R, Chen Y, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5:48.
Akturk H, Sutcu M, Somer A, et al. Carbapenem-resistant Klebsiella pneumoniae colonization in pediatric and neonatal intensive care units: risk factors for progression to infection. Braz J Infect Dis. 2016;20:134–40.
Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16:18.
Yan Z, Zhou Y, Du M, et al. Prospective investigation of carbapenem-resistant Klebsiella pneumonia transmission among the staff, environment and patients in five major intensive care units, Beijing. J Hosp Infect. 2019;101(2):150–7.
Yuan Y, Wang J, Yao Z, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infections and outcomes. Infect Drug Resist. 2020;13:207–15.
Ming-mei D, Yong Z, Zhen-guo S, et al. Effect of information technology on management of reasonable use of antibiotic in four years. Chin J Nosocomiol. 2016;26(18):4255.
Guo L, Wang L, Zhao Q, et al. Genomic analysis of KPC-2-producing Klebsiella pneumoniae ST11 isolates at the respiratory department of a tertiary care hospital in Beijing. China Front Microbiol. 2022;13:929826.
Yang J, Ye L, Guo L, et al. A nosocomial outbreak of KPC-2-producing Klebsiella pneumoniae in a Chinese hospital: dissemination of ST11 and emergence of ST37, ST392 and ST395. Clin Microbiol Infect. 2013;19(11):E509–15.
Acknowledgements
We thank the participants in the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Author Contributions
Conceptualization: Meng Li, Shanshan Yang, and Mingmei Du. Formal analysis and Investigation: Hongwu Yao and Yunxi Liu. Writing-original draft: Meng Li. All authors read and approved the final manuscript.
Disclosures
Meng Li, Shanshan Yang, Hongwu Yao, Yunxi Liu, and Mingmei Du have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Medical Ethics Committee of the Chinese. People’s Liberation Army (PLA) General Hospital (approval no. S2019-142-02) according to the Declaration of Helsinki. Informed consent was waived by the Medical Ethics Committee of the Chinese PLA General Hospital owing to the retrospective non-interventional study design. The data were anonymized before analysis.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, M., Yang, S., Yao, H. et al. Retrospective Analysis of Epidemiology, Risk Factors, and Outcomes of Health Care-Acquired Carbapenem-Resistant Klebsiella pneumoniae Bacteremia in a Chinese Tertiary Hospital, 2010–2019. Infect Dis Ther 12, 473–485 (2023). https://doi.org/10.1007/s40121-022-00732-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00732-7