Abstract
Patients with cardiac implantable electronic device (CIED) and Staphylococcus aureus bacteraemia (SAB) are at risk of having infective endocarditis (IE). The objectives were to describe a Swedish population-based cohort of patients with CIED and SAB, to identify risk factors, and to construct a predictive score for IE. Patients over 18 years old in the Stockholm Region identified to have SAB in the Karolinska Laboratory database from January 2015 through December 2019 were matched to the Swedish Pacemaker and Implantable Cardioverter-Defibrillator ICD Registry to identify the study cohort. Data were collected from study of medical records. A cohort of 274 patients with CIED and SAB was identified and in 38 episodes (14%) IE were diagnosed, 19 with changes on the CIED, and 35 with changes on the left side of the heart. The risk factors predisposition for IE, community acquisition, embolization, time to positivity of blood cultures, and growth in blood culture after start of therapy in blood cultures were independently associated to IE. A score to identify patients with IE was constructed, the CTEPP score, and the chosen cut-off generated a sensitivity of 97%, specificity of 25%, and a negative predictive value of 98%. The score was externally validated in a population-based cohort of patients with CIED and SAB from another Swedish region. We found that 14% of patients with CIED and SAB had definite IE diagnosed. The CTEPP-score can be used to predict the risk of IE and, when negative, the risk is negligible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac implantable electronic devices (CIEDs), including pacemaker (PM), implantable cardioverter defibrillator (ICD), cardiac resynchronization therapy-pacemaker (CRT-P), and cardiac resynchronization therapy-defibrillator (CRT-D), are used in a wide range of patients and are found in a growing cohort of patients [1]. When a patient with CIED has Staphylococcus aureus bacteraemia (SAB), the risk of CIED infection, pocket infection or infective endocarditis (IE), is high [2]. The diagnosis of CIED IE is based on positive blood cultures and visualization of vegetations or other structural changes indicating IE on echocardiography, transthoracic (TTE) or transoesophageal (TOE), positron emission tomography–computed tomography (PET-CT), or cardiac CT [2, 3]. In previous studies on the risk of IE in patients with SAB (Supplementary (S) Table 1) [4,5,6,7], CIED has been shown to be a risk factor [4, 6]. In patients with SAB and CIED, three risk factors, PM but not ICD, more than one device-related procedure, and growth in blood culture (BC) after start of therapy, have been found to predict IE [5] in a study from a tertiary referral centre.
The guidelines for treatment of CIED infections recommend extraction of the CIED in all cases of SAB due to the high risk of CIED infection [2], based on multiple studies, from tertiary centres, finding a high proportion of CIED infection in this category of patients (36–55%) [8,9,10,11]. However, the results from a recent population-based study showed that the rate of IE was only 19% and the risk of relapse in SAB was low despite short treatment times and that most patients were not subjected to CIED-extraction, indicating that missed CIED IE were few [12]. The authors suggested that in patients without pocket infection or changes on the CIED, extraction of the CIED might be omitted. Further, observations from studies have shown that patients with IE and CIED without changes on the CIED did not have the CIED extracted and was without relapse or other detrimental outcome [12, 13]. This implies that identification of patients with CIED changes is of interest. The discrepancy between the recommendations in the guidelines and the observations in these studies inspired us to further study patients with CIED and SAB.
The first aim was to describe a population-based cohort of patients with CIED and SAB, secondly to identify risk factors for CIED IE, and thirdly to develop a score predicting IE and to validate the score in an external cohort. Further, an aim was to evaluate risk factors for changes on the CIED. Finally, we wanted to suggest a management strategy for the patients.
Materials and methods
The cohort
The Swedish individual unique personal numbers were used to identify all consecutive patients with blood cultures (BC) positive for S. aureus from January 2015 to December 2019, obtained from the laboratory databases of Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden, with a catchment population of 1.9 million inhabitants. The patients’ identities were matched to the database of the Swedish Pacemaker and Implantable Cardioverter-Defibrillator ICD Registry (PMR), covering 97% of all pacemaker and ICD procedures in the Stockholm area [14]. Patients found in both registries were eligible to be included in the cohort. All patients with SAB older than 18 years with a CIED in place at the date of the positive BC constituted the study cohort. Data were collected by CC, validated by AB, and stored after ethical approval obtained from the Swedish Ethics Committee (2020–00314).
The validation cohort used in the study was the cohort from another region in Sweden, described in Berge et al. [12].
Definitions
The definition of CIED infection and CIED IE was from Blomström-Lundqvist et al. [2]. All infections fulfilling the criteria for definite IE were referred to as CIED IE irrespective whether changes were found on the CIED or on the heart valves [2, 15]. Any significant changes, compatible with IE, seen on TTE or TOE, were considered to constitute a major structural criterion for CIED IE, and the changes seen in anatomical association to the CIED were described as CIED changes [16]. An episode of SAB was defined by the start of the clinical symptoms or signs in a patient resulting in BC taken showing growth of S. aureus. To discriminate BC taken within an episode from a recurrent infection, an episode was delimited by at least 14 days of effective treatment and clinical improvement. A later clinical condition resulting in a positive BC with growth of S. aureus within the study period was referred to as a “recurrent infection” or “recurrence.” Growth after start of therapy was defined to be positive if any BC showed growth of S. aureus after ≥ 24 h of therapy. Origin of infection and other focal infections caused by S. aureus were defined as described [17]. Briefly, to fulfil the criteria for diagnosing a focal infection, two out of three criteria had to be present, culture showing growth of S. aureus from the site of infection, signs or symptoms from the site of infection, and imaging results compatible with the diagnosis. Comorbidities were retrieved from the medical records prior to the episode and classified according to the Charlson index modified by Quan et al. [18, 19].
Data collection and analysis
The collection of the microbiological data has been described (S material). Clinical data from each episode were collected from 365 days before the date of the first positive BC in the episode until 365 days after. The collected data from the patients’ medical records have been described (S material). The analysis of the collected data was conducted in Stata, version 15.1 (StataCorp, College Station, TX, USA). The odds ratios (OR) and their confidence intervals (CI) were calculated when applicable. The χ2-test was used when applicable, and otherwise, the p-value of Fisher’s exact test was used. Differences between continuous variables were analysed with Wilcoxon’s rank-sum test. Values have been presented as proportions in percent or medians with interquartile ranges (IQR). Multivariable logistic regression analyses (MVA) were done using forward stepwise regression. In some of the analyses, the “rule of ten” was disobeyed [20]. To be entered into the MVA, continuous variables, time to positivity in BC, age, and Charlson score, were dichotomized to best identify the outcome of the variables but prioritizing a high sensitivity. Dummy variables were created for different CIEDs and for place of acquisition. In the second MVA, a dummy variable was created for the non-nosocomial acquisition. Receiver operator characteristics (ROC) curves were constructed to identify the optimal cut off of both the dichotomized individual variables with continuous values and the scores. The area under the curve (AUC) and its confidence intervals were calculated. Variables significantly associated (p < 0.05) with the outcome in the univariable analysis were introduced into the MVAs, starting with the lowest p-value. Lack of data was replaced by zero in the data set, no other imputations were made, and no patients were lost in follow-up. The scores predicting IE in patients with SAB (PREDICT, PREDICT-SAB, VIRSTA, and POSITIVE) were calculated as described in the original publications [4,5,6,7], and the cut offs chosen by the authors were used.
Results
The cohort
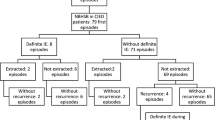
The search in the laboratory databases resulted in 8084 BCs positive for S. aureus in 3755 patients from January 2015 to December 2019. The same patients appeared in both databases in 359 of the SAB episodes. In 66 of the episodes, no CIED was in place at the time of the positive BC. In 293 episodes in 274 patients, the criteria were fulfilled for inclusion in the study, having a CIED when SAB occurred (Fig. 1). The first episodes of SAB in a patient, 274 episodes, were further studied. Definite IE was diagnosed in 38 patients. In 19 patients, changes were seen on the CIED and changes were seen on the left side of the heart in 35 patients. No patient was diagnosed with definite IE without structural changes constituting a major criterion. Changes on the CIED were found in five patients not fulfilling the diagnostic criteria of definite CIED IE. The five patients had only one positive BC and had less than three minor criteria and thus not classified as definite IE. Thirty-eight patients had the CIED extracted, 19 diagnosed with definite CIED IE, and 19 without IE diagnosis (Table 1). Nineteen recurrent episodes were found in 16 patients, two in patients with IE, and in 14 patients without IE during the first episode.
Comparison of clinical variables in patients with CIED IE and patients without CIED IE
Clinical variables in patients with and without CIED IE were analysed using univariate analysis. Significant differences were found in age, community acquisition, predisposition for IE, fever, embolization, growth in BCs fulfilling the major criterion for IE, time to positivity of BC, and growth in BC after start of therapy (Table 1). In 96 patients (35%), one or more BCs were taken after start of therapy. In the entire cohort, 115 patients (43%) had a TOE performed and 5 patients (2%) had a PET-CT. The median treatment time in the non-IE group was 14 days (10–25 days IQR). Mortality after 30 days was significantly lower in the IE group, 13%, compared to 31% in the non-IE group. After exclusion of patients not surviving the first 14 days from the start of the episode, no significant difference in mortality was seen (patients with IE, 8%, patients without IE, 9%, p-value 1.0, data not shown). The overall 365-day mortality was 54%, not significantly different between the groups (Table 1).
Variables independently associated to IE, receiver operator characteristics, and the scores
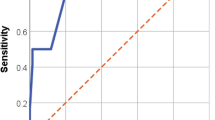
Variables significantly correlated to IE in univariate analysis were introduced into the MVA. The lowest number of outcomes per variable in the analysis was six (38 outcomes and six variables). Predisposition for IE, community acquisition, embolization, time to positivity of the BC less than 15 h, and growth in BC after start of therapy were independently correlated to IE (Table 2). The likelihood ratio test showed that the model without restrictions best fitted the data (p-value 0.017). Based on the OR of the MVA, each variable was given a value to be used in a score (Table 2). The score was given the name the CTEPP score from the first letter of each variable (community acquisition, time to positivity, embolization, predisposition for IE, and positive in BC after start of therapy). Based on the sum of the value given each variable from the MVA, a ROC curve was plotted (Fig. 2). The AUC was 0.79 (CI 0.71–0.87), significantly higher than the PREDICT-SAB score (0.51 (CI 0.41–0.61)) (Fig. 2).
To optimize the sensitivity and negative predictive value, but with a specificity as high as possible, a cut off for a positive result of the score of ≥ 2 was chosen resulting in a sensitivity of 97%, specificity of 25%, a negative predictive value of 98%, and the ratio between true positive and total numbers of positive was six. Analysis was made of the performance of the scores developed to assess the risk of IE in SAB patients (Table 3). PREDICT day 2 [5] and the POSITIVE score [7] significantly predicted IE and had a sensitivity of 76% and 58%, respectively, to identify patients with IE (Table 3). The other scores did not identify patients with IE from patients without IE significantly better than chance.
The validation cohort
The performance of the CTEPP score, to identify IE in patients with CIED and SAB, was tested in a validation cohort, a population-based cohort of the same category of patients with CIED and SAB from another region in Sweden [12]. The cohort consisted of 177 episodes of SAB, and IE was diagnosed in 25 episodes. The sensitivity of the CTEPP score to identify patients with IE was 100%, the specificity 13%, and the negative predictive value was 100% (data not shown).
Comparison of clinical variables between patients with CIED changes and patients without
To be able to identify risk factors associated with CIED changes found on any examination, a univariable analysis was performed on the Stockholm cohort using CIED changes as dependent variable. The analysis identified lower age, lower Charlson score, community and health care associated acquisition made into one variable, ICD, embolization, sepsis or septic shock at admission, pocket infection, short time to positivity of the BC, and growth in BC after start of therapy to be correlated to CIED changes (S Table 2).
In MVA, using forward stepwise regression, by first introducing the variable with the lowest p-value into the MVA, non-nosocomial acquisition (community and health care associated acquisition as one variable), ICD, embolization, and growth in BC after start of therapy were found to be independently correlated to CIED changes (Table 4). The likelihood ratio test showed that the model without restrictions best fitted the data (p-value < 0.001). The lowest number of outcomes per variable in the analysis was five (24 outcomes and five variables).
Discussion
In this study, we have described the clinical presentation of a population-based cohort of patients with CIED and SAB. Five variables, predisposition for IE, community acquisition, embolization, time to positivity in BCs less than 15 h, and growth in BCs after start of therapy, were identified as independent risk factors for IE and used in a score, the CTEPP score, with an excellent sensitivity to identify patients with CIED IE and with a high negative predictive value. We also found a low rate of CIED IE, 14%, in concordance with a recently published population-based report [12], but in contrast to previous reports [8,9,10,11], all from tertiary referral centres, that found much higher rates (36–55%).
Some studies during the last years have studied risk factors for IE in patients with SAB [4,5,6,7] but have identified different variables (S Table 1). Previously, only one study, the PREDICT-SAB study, from a tertiary referral centre and not population-based, has focused on risk factors for IE in patients with CIED [5], being the primary comparison to the results of this study. The study identified PM, more than one device-related procedure, and growth in BC after more than 3 days after start of therapy (given the description “prolonged SAB” and “SAB ≥ 4 days”) to be independently correlated to IE. Only growth in BC after start of therapy appeared in our study too, although defined differently. An explanation of the diverse findings could be the composition of the cohorts; our study being population-based making it more likely to be generalizable. Another striking difference between some other studies and our study is the rate of methicillin resistant S. aureus (MRSA) (40% and 2%, respectively) [5]. The rate of MRSA in our study was not different to the rate of MRSA in all BCs in the region. The difference in rate could likely be due to the selection of infections difficult to treat, caused by MRSA, in referral centres. All the studies have the weakness of being performed with retrospective data.
The international guidelines have recommended TOE to be performed in all cases of patients with CIED and SAB and to continue with further evaluation of the patient with repeated TOEs, PET-CT, or cardiac CT, if the suspicion of IE remains. In our study, the risk of CIED IE was low with a negative CTEPP score. We propose that in all patients with CIED and SAB, TOE should be performed, but also suggest that further evaluation could be omitted after both a negative TOE and a negative CTEPP score.
To address the question on how to identify patients with CIED changes, we have shown that some of the risk factors associated with CIED changes were the same and some differ from those we identify to be associated to IE (Tables 2 and 4), although the episodes overlap to a large extent in the present study (19 episodes of 43, 44%; Table 1). Community or health care associated acquisition, ICD, embolization, and growth in BCs after start of therapy were independently correlated to CIED changes. This indicates the biologically plausible hypothesis that risk factors differ between IE and CIED changes. We have refrained from presenting yet another score to predict CIED changes although it performed very well and could be valuable if extraction was to be performed only if CIED changes were found.
This study has several limitations. The first being the retrospective design limiting the acquisition of a full data set. In a limited proportion, 42% of the study cohort, TOE was performed, introducing the risk that some patients with CIED IE could have been misclassified. In the PREDICT-SAB study, 64% of the patients in the study population were examined with TOE [5]. The decision whether to perform a TOE in patient with SAB was decentralized to the treating doctors, their assessment of the risk of CIED IE, and the individual situation of the patient, probably explaining the low proportion of TOE performed. Further, in the group of patients that died within 14 days, the IE diagnosis was rare and can possibly contain misclassified cases. However, the median treatment time (14 days) and the limited number of relapses (6%) suggest that the number of misclassified patients was low. The MVA identified growth after start of therapy to be independently correlated to CIED IE, but only 35% of the patients were re-cultured. A prospective dataset would overcome these shortcomings.
The plethora of scores in patients with SAB can be confusing; therefore, a prospective multicentre study, collecting data from the identified risk factors in all the studies and other variables of interest, would have the possibility to illuminate this complicated clinical situation. A prospective validation study of three of the scores found the VIRSTA score to have the best performance [21] in a cohort of all patients with SAB. The study did not have the aim to identify previous known risk factors or new ones and the cohort only contained 11% CIED carriers. Further, both PREDICT-SAB and the present study indicated that the risk factor profile is different between SAB episodes in patients with CIED and SAB episodes in patients without CIED. Finally, identification of patients with and without CIED IE and with and without changes on the actual leads, CIED changes, may be the foundation for exciting studies on in what situations extraction of the CIED should be recommended. Thus, the results of prospective multicentre studies of CIED and SAB cohorts would be most interesting. Waiting for those studies, we think that the clinician can be helped by the results of this study, the largest study on risk factors for IE in patients with CIED and SAB so far, to assess the risk CIED IE.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ (2015) 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 36(41):2793–2867
Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, Poole J, Boriani G, Costa R, Deharo JC, Epstein LM, Saghy L, Snygg-Martin U, Starck C, Tascini C, Strathmore N (2020) European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 57(1):e1–e31
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, Group ESCSD (2015) 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36(44):3075–3128
Palraj BR, Baddour LM, Hess EP, Steckelberg JM, Wilson WR, Lahr BD, Sohail MR (2015) Predicting Risk of Endocarditis Using a Clinical Tool (PREDICT): Scoring System to Guide Use of Echocardiography in the Management of Staphylococcus aureus Bacteremia. Clin Infect Dis 61(1):18–28
Sohail MR, Palraj BR, Khalid S, Uslan DZ, Al-Saffar F, Friedman PA, Hayes DL, Lohse CM, Wilson WR, Steckelberg JM, Baddour LM (2015) Predicting risk of endovascular device infection in patients with Staphylococcus aureus bacteremia (PREDICT-SAB). Circ Arrhythm Electrophysiol 8(1):137–144
Tubiana S, Duval X, Alla F, Selton-Suty C, Tattevin P, Delahaye F, Piroth L, Chirouze C, Lavigne JP, Erpelding ML, Hoen B, Vandenesch F, Iung B, Le Moing V, Group VAS (2016) The VIRSTA score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with Staphylococcus aureus bacteremia. J Infect 72(5):544–553
Kahn F, Resman F, Bergmark S, Filiptsev P, Nilson B, Gilje P, Rasmussen M (2021) Time to blood culture positivity in Staphylococcus aureus bacteraemia to determine risk of infective endocarditis. Clin Microbiol Infect: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 27(9):1345.e1347–1345.e1312
Chamis AL, Peterson GE, Cabell CH, Corey GR, Sorrentino RA, Greenfield RA, Ryan T, Reller LB, Fowler VG (2001) <b><i>Staphylococcus aureus</i> Bacteremia in Patients With Permanent Pacemakers or Implantable Cardioverter-Defibrillators</b>. Circulation 104(9):1029–1033
Uslan DZ, Dowsley TF, Sohail MR, Hayes DL, Friedman PA, Wilson WR, Steckelberg JM, Baddour LM (2010) Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol 33(4):407–413
Maskarinec SA, Thaden JT, Cyr DD, Ruffin F, Souli M, Fowler VG (2017) The risk of cardiac device-related infection in bacteremic patients is species specific: results of a 12-year prospective cohort. Open Forum Infect Dis 4(3):ofx132
Chesdachai S, Baddour LM, Sohail MR, Palraj BR, Madhavan M, Tabaja H, Fida M, Lahr BD, DeSimone DC (2022) Evaluation of European Heart Rhythm Association consensus in patients with cardiovascular implantable electronic devices and Staphylococcus aureus bacteremia. Heart Rhythm 19(4):570–577
Berge A, Strand R, Nilson B, Naucler P, Rasmussen M (2022) Staphylococcus aureus bacteremia, cardiac implantable electronic device, extraction, and the risk of recurrence. J Infect 84(5):e67–e69
Iversen K, Ihlemann N, Gill SU, Madsen T, Elming H, Jensen KT, Bruun NE, Hofsten DE, Fursted K, Christensen JJ, Schultz M, Klein CF, Fosboll EL, Rosenvinge F, Schonheyder HC, Kober L, Torp-Pedersen C, Helweg-Larsen J, Tonder N, Moser C, Bundgaard H (2019) Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N Engl J Med 380(5):415–424
Gadler F, Valzania C, Linde C (2015) Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace 17(1):69–77
Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR (2000) Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30(4):633–638
George MP, Esquer Garrigos Z, Vijayvargiya P, Anavekar NS, Luis SA, Wilson WR, Baddour LM, Sohail MR (2021) Discriminative ability and reliability of transesophageal echocardiography in characterizing cases of cardiac device lead vegetations versus noninfectious echodensities. Clin Infect Dis 72(11):1938–1943
Berge A, Krantz A, Ostlund H, Naucler P, Rasmussen M (2019) The DENOVA score efficiently identifies patients with monomicrobial Enterococcus faecalis bacteremia where echocardiography is not necessary. Infection 47(1):45–50
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V (2011) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173(6):676–682
Vittinghoff E, McCulloch CE (2006) Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 165(6):710–718
van der Vaart TW, Prins JM, Soetekouw R, van Twillert G, Veenstra J, Herpers BL, Rozemeijer W, Jansen RR, Bonten MJM, van der Meer JTM (2022) Prediction rules for ruling out endocarditis in patients with Staphylococcus aureus bacteremia. Clin Infect Dis 74(8):1442–1449
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ (2002) Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137(10):791–797
Bizzini A, Durussel C, Bille J, Greub G, Prod’hom G (2010) Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol 48(5):1549–1554
Vondracek M, Sartipy U, Aufwerber E, Julander I, Lindblom D, Westling K (2011) 16S rDNA sequencing of valve tissue improves microbiological diagnosis in surgically treated patients with infective endocarditis. J Infect 62(6):472–478
Johansson N, Vondracek M, Backman-Johansson C, Sköld MC, Andersson-Ydsten K, Hedlund J (2019) The bacteriology in adult patients with pneumonia and parapneumonic effusions: increased yield with DNA sequencing method. Eur J Clin Microbiol Infect Dis 38(2):297–304
Acknowledgements
We are indebted to Mrs. Anita Fredenson, Karolinska University Hospital, Stockholm, Sweden, and Mr. Zsolt Palfi for invaluable help to match the Swedish Pacemaker and Implantable Cardioverter-Defibrillator Registry to the Microbiology data to identify the cohort of the study.
Funding
Open access funding provided by Karolinska Institute. The study was also supported by the Region Stockholm (ALF grant) for AB.
Author information
Authors and Affiliations
Contributions
AB and MR are responsible for the study conception. All authors contributed to the study design. Data collection was performed by CC under the supervision of AB, and AB did the data analysis. The first draft of the manuscript was written by AB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Data were collected and stored after ethical approval obtained from the Swedish Ethics Committee (2020–00314).
Consent to participate
According to the Ethics Approval, no consent to participate was needed.
Consent for publication
Not applicable (see above).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berge, A., Carlsén, C., Petropoulos, A. et al. Staphylococcus aureus bacteraemia, cardiac implantable electronic device, and the risk of endocarditis: a retrospective population–based cohort study. Eur J Clin Microbiol Infect Dis 42, 583–591 (2023). https://doi.org/10.1007/s10096-023-04585-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04585-x