Abstract
Patients with brain injury are prone to bacterial colonisations because of mechanical ventilation during intensive care and the long-term retention of tracheostomical tubes during rehabilitation. Reduced levels of isolation, typical of rehabilitation, could also contribute to propagate colonisations. We evaluated the presence of bacteria through different stages of healthcare, their antibiotic resistances and their clinical impact in a rehabilitation setting. This retrospective study included all tracheostomised patients referred to the paediatric brain injury unit of the Scientific Institute IRCCS E. Medea (Italy) over a six-year period. Data were collected from antibiograms regarding the presence of bacterial species and antibiotic resistances; clinical data were collected from medical records. Antibiograms revealed bacteria and antibiotic resistances typical of intensive care, while prevalence patterns were characteristic for each species (P. aeruginosa and S. aureus prevailing in the acute setting, K. pneumoniae, A. baumannii and others in rehabilitation). Despite very frequent antibiotic resistances, consistent with Italian averages, we observed a limited clinical impact for these colonisations. We analysed risk factors correlating to the development of respiratory symptoms and found a role for the acute clinical course after brain injury (having undergone neurosurgery; duration of intensive care stay) as well as for rehabilitation (duration of coma). Our data suggest that, in a long-term perspective, an appropriate balance is yet to be found between patient isolation and social interactions, to control respiratory colonisations and antibiotic resistances without compromising rehabilitation. They also suggest that regular containment measures should be complemented by thorough training to non-medic personnel and parents alike.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2012 report of the European Centre for Disease Control [1] showed an alarming prevalence of antibiotic resistance in several countries, including Italy, due to bacteria frequently associated with nosocomial pneumonia [2]. These bacteria are common in adult and paediatric intensive care units (ICUs) [3, 4] and have been associated with increased mortality rates and longer length of stay [5]. Such issues frequently involve patients with acquired brain injury (ABI) [6], who present several risk factors such as mechanical ventilation, long-lasting impairment of consciousness and having undergone surgery [7, 8]. While mechanical ventilation during short-term hospitalisation in ICUs is associated with higher risk of developing pneumonia [9] and containment protocols are widely discussed [10, 11], information about long-term care of patients with tracheostomical tubes is scant. Patients with ABI often maintain tracheostomies out of ICUs, due to neurological deficiencies [12–14], and their presence has been associated with higher prevalence of pneumonia [15]. At the same time these patients are exposed to intense interactions with healthcare professionals and relatives who may be a prominent source of pathogens [16–19]. The adequate management [20, 21] of nosocomial pneumonia sustained by antibiotic-resistant bacteria is important for the consequences both on the clinical management choices for patients with ABI in ICUs [22] and for their long-term need of medical treatments [23]. To investigate these issues in a long-term perspective, we analysed a population of paediatric tracheostomised patients undergoing rehabilitation from ABI, focusing on the presence of bacteria during different stages of healthcare, their spectrum of antibiotic resistance and the development of respiratory symptoms.
Materials and methods
Study design and settings
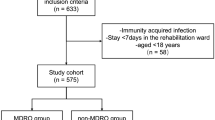
The Scientific Institute IRCCS Eugenio Medea is a paediatric clinic for neurological and neuropsychiatric rehabilitation. It hosts a Severe ABI Unit that accepts patients with post-acute brain injury of various aetiologies. This retrospective cohort study evaluated medical records from the 2007–2013 time frame, focusing only on patients who suffered ABI and carried a tracheostomical tube at admission, including all available microbiological screenings performed until tracheostomy was removed. Patients were initially admitted from acute-care health facilities for their first rehabilitation stay, during which patients continuously resided in the unit. As rehabilitation usually lasts several years, patients re-entered the unit for multiple follow-up stays of variable duration; therefore, after the first discharge from the unit, patients had uncontrolled interactions with the community and other healthcare structures. Whether admitted for the first rehabilitation stay or for follow-ups, patients in the unit were hosted in non-isolated single rooms, with the full-time presence of a relative. General hygienic measures, including hand washing and clothing change, were regularly taught to parents, while further containment measures, such as prevention of contact and airborne contaminations, were only applied in cases of virulent or drug resistant bacteria. During each stay patients received neurological, cognitive and physical rehabilitation, comprising regular sessions with mechanical in/ex-sufflators to clear bronchial secretions. Patients were pharmacologically treated for spasticity, autonomic imbalances and pain; several antibiotic treatments were prescribed for relevant symptoms of respiratory, urinary, topical and central infections following medical judgment.
Data collection
Data regarding patient characteristics, medical interventions and complications were collected from clinical records. These comprised: gender, ABI aetiology, patient provenance, past neurosurgery, presence of paroxysmal sympathetic hyperactivity, presence of bedsores; scores relative to GOS, GCS, DRS scales; date of birth, injury, admission to rehabilitation, discharge and emersion from coma. Dates were elaborated to obtain the age of patients at ABI (date of ABI – date of birth), the time between ABI and admission to this rehabilitation unit (date of admission – date of ABI) and the duration of coma (date of emersion from coma – date of ABI; for patients who did not recover consciousness, duration of coma was levelled to the duration of follow-up); presence of central venous catheter, bladder catheter, ventricular-peritoneal shunt and feeding devices. Microbiological data were collected from antibiograms, as described in the next section. For sample stratification patients were considered as suffering from respiratory complications if any single criterion was met from the American CDC guideline for the diagnosis of ventilator-associated pneumonia [24]. Patients were assessed for the presence of respiratory symptoms daily during the rehabilitative stay and also during the follow-up visits. In this case, both the presence of acute symptoms and the presence of positive medical records contributed to the definition of symptoms. Patient consents regarding the use of anonymised data for research purposes were obtained. This study was notified to the local Ethics Committee and approved according to the Italian rules.
Microbiological screening and sampling
At admission all patients were screened to detect pathogens, while regular screenings were subsequently carried out which ranged from one per month in patients with no evident symptom and no antibiotic therapy, to one per week, in patients with symptoms and on-going antibiotic therapies. In order to perform the screenings, samples of tracheal secretions were collected by aspiration, using a sterile probe and following standard medical procedures. Samples were processed with Sputasol reagent (Oxoid) and cultured in the structure’s microbiology laboratory using precast culture plates (bioMérieux). Cultures were identified and sensitivity to antibiotics was tested using the Vitek 2 automated platform (bioMérieux). Data were collected from antibiograms, regarding dates, bacterial loads and resistances to antibiotics. Colonisations were classified as acquired before rehabilitation (i.e. present at patient’s admission), acquired during the first rehabilitation stay (i.e. acquired in the unit), or acquired during follow-up (i.e. acquired in healthcare or community/uncertain source). A patient was considered carrying a resistant bacterium if resistance was ever detected, thus including bacteria that became resistant or were replaced by resistant strains. Bacteria were categorised for antibiotic-class resistances following criteria available from the literature [25].
Statistical methods
Patients were subdivided with respect to the presence of respiratory complications and differences between the two groups were tested with χ2 (categorical variables) or analysis of variance (continuous variables). χ2 tests were also applied to the numbers of patients who acquired colonisations during the different stages of healthcare. p values < 0.05 were considered to be significant and higher p values were considered for possible trends. All statistical analyses were performed using MedCalc® (Version 12.7.8 MedCalc Software bvba).
Results
Risk factors for respiratory symptoms development
In the 2007–2013 time-frame, 65 patients carrying a tracheostomical tube were referred to our Institute for rehabilitation after ABI. Of these, 33 (50.8 %) developed respiratory symptoms. Table 1 describes the main clinical-demographical characteristics and possible risk factors of the patients under study. No significant relationship was found between any of these factors and the presence of respiratory complications in the cohort, although a non-significant difference was noted for several factors, between the symptomatic and asymptomatic groups. Symptomatic patients underwent acute neurosurgery after ABI more than asymptomatic ones (69.7 % vs. 46.2, p = 0.10). In symptomatic patients, the time between ABI and admission to rehabilitation was 50 % longer (110 ± 108 vs. 72 ± 50 days, p = 0.10) and the duration of coma was 23 % lower (122 ± 75 vs. 159 ± 92 days, p = 0.11).
Respiratory colonisation by bacterial strains
Most patients (91 %) had airways already colonised by bacteria at admission to our unit, a value reaching 94 % during the first rehabilitation stay and virtually 100 % during follow-up (1 patient only remaining persistently free of colonisations). In addition, during the first rehabilitation stay, 23 % of the patients acquired one additional respiratory colonisation and 26 % of them two or more. The numbers of patients carrying each bacterium were considered during three stages of healthcare, corresponding to the acute care preceding rehabilitation, the rehabilitation stay in the unit and finally the follow-up period in an uncontrolled setting, as long as patients still carried tracheostomical tubes (Table 2). S. aureus was the most frequently detected bacterium (in 73.8 % of the patients) and showed a statistically significant difference in its prevalence pattern; it was mostly found in patients before the first entry for rehabilitation (66.7 %, p < 0.01) and patients who acquired it, did so predominantly in the unit (27.1 % vs. 6.3 %, p < 0.01). P. aeruginosa was also frequent (in 72.3 % of the patients) and preferentially acquired before the first admission for rehabilitation ( in 59.6 % of the cases p = 0.14). By contrast, K.pneumoniae, detected in 32.3 % of the patients, was acquired significantly more during rehabilitation (81 %, p < 0 .01) and in the unit than during follow-up (52.4 % vs. 28.6 %, p = 0.02). E. coli and A. baumannii, affecting 20 % and 18.5 % of the patients, respectively, were acquired mostly during rehabilitation (E. coli in 76.9 % of the cases, p = 0.08; A. baumannii 83.3 %, p = 0.03), both inside and outside the unit. S. marcescens was rarely detected (in 12.3 % of the patients) and showed no significant prevalence pattern, while S. capitis, affecting only 9.2 % of the patients, was never recorded present before the first admission for rehabilitation (p = 0.03) and developed essentially in the unit (in 83.3 % of the cases) (p = 0.02). Less frequently diagnosed bacteria (data not shown) were S. maltophilia, K. oxytoca, M. morganii, other Enterobacteriaceae and other Staphylococci. S. pneumoniae was only detected in two patients, at one visit. No consistent seasonal pattern for colonisations was found. A graphical representation of the distribution of S. aureus and P. aeruginosa colonisations is available in Supplementary Fig. 1.
Antibiotic resistance of bacterial strains
Antibiotic resistance was frequently detected across bacteria from different patients (Table 3). P. aeruginosa was found to be resistant in 80.9 % of the affected patients, mostly to carbapenems and cephalosporins, followed by fluoroquinolones and aminoglicosides; 27.7 % of these patients carried multiresistant variants. A. baumannii proved to be resistant in 58.3 % of the patients with multiresistant strains in 33.3 % of the cases. MRSA was detected in 54.2 % of the patients carrying S. aureus; of these, only a minority resisted also to vancomycin (8.3 %). S. capitis was always found resistant to methicillin and never to vancomycin. E. coli was resistant in 53.8 % of the patients and ESBL-like activity had a 38.5 % prevalence; resistance to fluoroquinolones was more frequent (46.2 %) and one isolated case of carbapenems resistance was found. K. pneumoniae showed a similar profile and two KPC cases were detected. S. marcescens displayed resistance to carbapenems in one patient and ESBL-like activity in two.
Discussion
Respiratory colonisations and infections represent a relevant source of morbidity for patients who suffered ABI, both in the ICU and during the subsequent rehabilitation [22]. Care for these patients is often complicated by antibiotic resistance, especially in countries where it occurs frequently [1]. All these factors have a significant clinical and economic impact in both short- and a long-term perspectives [23]. Thus, knowing the characteristics and relative burden of bacteria residing in a group of patients in one hospital unit may help identifying appropriate strategies to minimise their clinical impact. In terms of clinical variables and possible risk factors, data collected from our patients did not significantly associate with the emergence of respiratory symptoms; however, a trend of association for some of them was observed. In this respect it must be emphasised that the cohort of patients in this study is not large which affects the power of the study. The only medical procedure possibly associated with the presence of respiratory symptoms was neurosurgery, performed acutely after ABI, which had already been linked to an increased risk of respiratory infections in ICUs [13]. The time between ABI and rehabilitation was longer in symptomatic patients and may indicate a more severe clinical course in intensive care; this is consistent with previous work that associated longer mechanical ventilation in ICUs to a higher risk of ventilator-associated pneumonia [9]. An intriguing finding is that duration of coma was lower in symptomatic patients; as patients emerge from coma they get in closer contact with more relatives and friends, and also become eligible for advanced rehabilitation procedures, with operators that are not used to treating fragile patients. This represents a clinically relevant risk, directly related to the rehabilitation process. In this respect, the fact that rehabilitation can last for years and patients get in contact with other people in a non-controlled environment during this period, is indeed a limitation of this study. Some symptomatic cases could be caused by viral infections and, thus, not related to bacterial colonisation. These, however, were not investigated in the clinical setting we described. From the beginning of the rehabilitation to the end of follow-up the overall prevalence of respiratory colonisations increased only slightly. Involved bacteria were those typical of nosocomial respiratory infections [2], in terms of species and respective prevalence. S. aureus and P. aeruginosa showed very similar prevalence among patients (around three quarters), followed by K. pneumoniae with almost one third of the patients affected and E. coli with one fifth, while others were less represented. Importantly, more than one fourth of patients acquired more than one respiratory colonisation, a course completely different from the one commonly observed in ICUs [11]. We found interesting differences in the prevalence pattern of different species. S. aureus and P. aeruginosa colonisations mostly entered the rehabilitation unit from acute care settings, i.e. ICUs and neurosurgery units. Furthermore, the distribution of S. aureus showed a clear preference for protected nosocomial settings, as the number of newly colonised patients was maximal before rehabilitation, then decreased in the rehabilitation unit, and again decreased during the follow-up. This suggests two possible interpretations, first, that the role of physicians and nurses may be crucial for propagating S. aureus, and second, that as patients recover from TBI, acquisition of S. aureus becomes less probable. In a partially similar fashion, P. aeruginosa showed a trend for association with the period preceding rehabilitation, possibly indicating a tighter relation to patients’ general severity. A different pattern was observed for K. pneumoniae and S. capitis that were acquired mostly during rehabilitation, with the first generally inside the unit, the second exclusively. This suggests an important influence of factors typical of rehabilitation, including intense interactions with healthcare professionals [26, 27] and relatives. While guidelines for preventing nosocomial infections are available to physicians and nurses [20, 21], no such measure is officially available for therapists, such as logopaedists and physiatrists; furthermore, parents may carry pathogens from patient to patient [17–19]. This attitude is currently dependent on personal expertise, and untrained operators or relatives could nullify the benefits of single rooms in reducing the nosocomial transmission of pathogens, as shown in ICUs [28, 29]. Finally, it should be emphasised that only symptomatic patients were receiving antibiotics during rehabilitation (data not shown) and this may possibly lead to more bacterial colonisations. Interestingly, S. pneumoniae was detected only twice in our cohort, at variance with other studies that reported higher prevalence in long-term tracheostomised patients [30]. The relative prevalence we found among species was in fact very similar to the one described in a recent review on nosocomial pneumonia [2]; possible explanations of discrepancies in the reported prevalence of S. pneumoniae may comprise, but are not limited to, different geographical locations (involving different provenance hospitals and environments) and treatment habits, both before and during rehabilitation.
In terms of resistance to antibiotics we found that P. aeruginosa was extensively resistant to carbapenems (two thirds of patients), and S. aureus resistant to methicillin in more than half of the patients, but uncommonly resistant to vancomycin (8.3 %). The highly prevalent resistance to fluoroquinolones may likely be related to the very high occurrence of urinary tract infections, and consequent antibiotic usage, in patients who suffered ABI (data not shown). Carbapenemase-producing strains were detected in two patients for K. pneumoniae and in one patient respectively for E. coli and S. marcescens, with no case of colonisation acquired in the unit. Such strains, currently emerging in southern Europe, entered the unit over the last two consecutive years and represent a warning bell for the future. As this resistance is easily acquired, special containment measures were enforced to prevent outbreaks [31], such as partial room isolation and whole unit screening, with success. Other bacteria were detected in numbers too small to allow specific comparison to large-scale studies. Of importance, data we report here are the first ones describing antibiotic resistances in a long-term ABI rehabilitation setting; available information to date was either in the form of aggregate data from ECDC [1] or from ICUs.
Overall, the picture of a paediatric rehabilitation unit we report on describes a setting where a balance must be constantly found, between the primary medical objective and related complications; rehabilitation itself is a risk factor for colonisation and infection, as social interaction and contact are encouraged, while isolation is not feasible. This is supported by the vast prevalence of bacterial colonisations that we described, which increased further during rehabilitation. However, it is possible to moderate the spreading of bacteria by applying adequate care measures on a flexible basis. Examples in the specific setting we described include the administration of antibiotics only to symptomatic patients, in order to minimise the development of resistances, and the adaptation of hygiene and containment levels to each patient’s need. In order to implement these measures, training is regularly given to parents and operators (e.g. hand hygiene, clothing change and personal protection); furthermore, when multi-resistant bacteria are identified, additional care measures are implemented, such as protection from droplets and airborne contaminants, with attention to each peculiar pathogen [20, 21, 31]. Unfortunately compliance of operators from other units and parents to the suggested measures cannot be monitored. However, the clinical impact of respiratory colonisations was overall irrelevant; moreover, no patient needed to be transferred to primary care facilities for respiratory complications. Therefore, even if implemented measures are yet far from full efficiency, the chosen approaches proved to be valuable at a local level.
References
ECDC (2013) Antimicrobial resistance surveillance in Europe 2012. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net), Stockholm
Nair GB, Niederman MS (2013) Nosocomial pneumonia: lessons learned. Crit Care Clin 29(3):521–546
Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD et al (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302(21):2323–2329
Tekin R, Dal T, Pirinccioglu H, Oygucu SE (2013) A 4-year surveillance of device-associated nosocomial infections in a neonatal intensive care unit. Pediatr Neonatol 54(5):303–308
Foglia EE, Fraser VJ, Elward AM (2007) Effect of nosocomial infections due to antibiotic-resistant organisms on length of stay and mortality in the pediatric intensive care unit. Infect Control Hosp Epidemiol 28(3):299–306
Scott BN, Roberts DJ, Robertson HL, Kramer AH, Laupland KB, Ousman SS et al (2013) Incidence, prevalence, and occurrence rate of infection among adults hospitalized after traumatic brain injury: study protocol for a systematic review and meta-analysis. Syst Rev 2(1):68
Alharfi IM, Stewart TC, Helali IA, Daoud H, Fraser DD (2013) Infection rates, fevers, and associated factors in pediatric severe traumatic brain injury. J Neurotrauma 31(5):452–458
Sopena N, Heras E, Casas I, Bechini J, Guasch I, Pedro-Botet ML et al (2014) Risk factors for hospital-acquired pneumonia outside the intensive care unit: A case-control study. Am J Infect Control 42(1):38–42
Hui X, Haider AH, Hashmi ZG, Rushing AP, Dhiman N, Scott VK et al (2013) Increased risk of pneumonia among ventilated patients with traumatic brain injury: every day counts! J Surg Res 184(1):438–443
Ramirez P, Bassi GL, Torres A (2012) Measures to prevent nosocomial infections during mechanical ventilation. Curr Opin Crit Care 18(1):86–92
Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA et al (2012) International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control 40(5):396–407
Mitchell RB, Hussey HM, Setzen G, Jacobs IN, Nussenbaum B, Dawson C et al (2013) Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg 148(1):6–20
Citta-Pietrolungo TJ, Alexander MA, Cook SP, Padman R (1993) Complications of tracheostomy and decannulation in pediatric and young patients with traumatic brain injury. Arch Phys Med Rehabil 74(9):905–909
Stelfox HT, Crimi C, Berra L, Noto A, Schmidt U, Bigatello LM, Hess D (2008) Determinants of tracheostomy decannulation: an international survey. Crit Care 12(1):R26
Hansen TS, Larsen K, Engberg AW (2008) The association of functional oral intake and pneumonia in patients with severe traumatic brain injury. Arch Phys Med Rehabil 89(11):2114–2120
Danzmann L, Gastmeier P, Schwab F, Vonberg RP (2013) Health care workers causing large nosocomial outbreaks: a systematic review. BMC Infect Dis 13:98
Cocchi P, Taccetti G, Montagnani C, Campana S, Galli L, Braggion C, de Martino M (2013) Evidence of transmission of a Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus clone: a family affair. Clin Microbiol Infect 19(12):1158–1162
Cartolano GL, Moulies ME, Seguier JC, Boisivon A (2003) A Parent as a vector of Salmonella brandenburg nosocomial infection in a neonatal intensive care unit. Clin Microbiol Infect 9(6):560–562
Morel AS, Wu F, Della-Latta P, Cronquist A, Rubenstein D, Saiman L (2002) Nosocomial transmission of methicillin-resistant Staphylococcus aureus from a mother to her preterm quadruplet infants. Am J Infect Control 30(3):170–173
Siegel JD, Rhinehart E, Jackson M, Chiarello L (2007) 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 35:S65–S164
WHO. Guidelines on hand hygiene in health care (2009). Available at: http://www.who.int/iris/bitstream/10665/78060/1/9789241503372_eng.pdf
Yang CC, Shih NC, Chang WC, Huang SK, Chien CW (2011) Long-term medical utilization following ventilator-associated pneumonia in acute stroke and traumatic brain injury patients: a case-control study. BMC Health Serv Res 11:289
Wilke M, Grube R (2013) Update on management options in the treatment of nosocomial and ventilator assisted pneumonia: review of actual guidelines and economic aspects of therapy. Infect Drug Resist 7:1–7
CDC (2014) Manual on the surveillance for ventilator-associated pneumonia (VAP) events. Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf. Accessed 13/02/2014
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281
Boque MC, Bodi M, Rello J (2000) Trauma, head injury, and neurosurgery infections. Semin Respir Infect 15(4):280–286
Dziedzic T, Slowik A, Szczudlik A (2004) Nosocomial infections and immunity: lesson from brain-injured patients. Crit Care 8(4):266–270
Ben-Abraham R, Keller N, Szold O, Vardi A, Weinberg M, Barzilay Z, Paret G (2002) Do isolation rooms reduce the rate of nosocomial infections in the pediatric intensive care unit? J Crit Care 17(3):176–180
Bracco D, Dubois MJ, Bouali R, Eggimann P (2007) Single rooms may help to prevent nosocomial bloodstream infection and cross-transmission of methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med 33(5):836–840
Adler A, Ben-David D, Schwaber MJ, Masarwa S, Schwartz D, Porat N et al (2012) Prevalence of Streptococcus pneumoniae in respiratory samples from patients with tracheostomy in a long-term-care facility. J Clin Microbiol 50(10):3368–3370
CDC (2012) CRE Toolkit - Guidance for Control of Carbapenem-resistant Enterobacteriaceae (CRE). Available at: http://www.cdc.gov/hai/organisms/cre/cre-toolkit/index.html?s_cid=fb2214. Last accessed 13/02/2014
Acknowledgments
The financial support by Agenzia Italiana del Farmaco (AIFA) and the Italian Ministry of Health (Ricerca Corrente 2014, to EC and SS) is gratefully acknowledged. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Marco Pozzi and Paolo Pellegrino authors contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 128 kb)
Rights and permissions
About this article
Cite this article
Pozzi, M., Pellegrino, P., Galbiati, S. et al. Prevalence of respiratory colonisations and related antibiotic resistances among paediatric tracheostomised patients of a long-term rehabilitation centre in Italy. Eur J Clin Microbiol Infect Dis 34, 169–175 (2015). https://doi.org/10.1007/s10096-014-2220-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2220-x