Abstract
The purpose of this study was to assess the main clinical predictors and microbiological features of ventilator-associated pneumonia (VAP) in the Intensive Care Unit (ICU) environment. This work is a retrospective analysis over one year from September 2010 to September 2011. Patients’ risk factors, causes of admission, comorbidities and respiratory specimens collected in six Italian ICUs were reviewed. Incidence and case fatality rate of VAP were evaluated. After stratification for VAP development, univariate and multivariate analyses were performed to assess the impact of patients’ conditions on the onset of this infection. A total of 1,647 ICU patients (pts) were considered. Overall, 115 patients (6.9 %) experienced at least one episode of VAP. The incidence rate for VAP was 5.82/1,000 pts-days, with a case fatality rate of 44.3 %. Multivariate analysis showed that admission for neurological disorders (aIRR 4.12, CI 1.24–13.68, p = 0.02) and emergency referral to ICU from other hospitals (aIRR 2.11, CI 1.03–4.31, p = 0.04) were associated with higher risk of VAP, whereas a tendency to a higher risk of infection was detected for admission due to respiratory disease, cardiac disease, trauma and for having obesity or renal failure. A total of 372 microbiological isolates from respiratory specimens were collected in VAP patients. The most common species were Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa, showing high resistance rates to carbapenems. Neurological disorders and emergency referral at the admission into the ICU are significantly associated with the onset of VAP. A high incidence of multi-drug resistant Gram- species was detected in the respiratory specimens.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is currently recognized as one of the most relevant causes of morbidity and mortality among intensive care unit (ICU) patients worldwide and is estimated to occur in 8 % to 28 % of all mechanically ventilated patients [1]. In Europe, a burden of 18,900 cases of VAP have been estimated to occur each year [2], with a mortality rate up to 50 % of cases in some reports and as high as 76 % in the most compromised patients, especially when multidrug-resistant pathogens are involved [3, 4]. At the present time, VAP is considered to be the second most frequent nosocomial infection occurring in the ICU after central lines infections, and it may account for up to 25 % of all hospital-acquired infections in the ICU environment [1]. According to the ATS/IDSA guidelines, VAPs can be classified as early-onset VAP or late-onset VAP depending on whether they occur within or after the first 96 hours of hospitalization, respectively [3].
The main risk factors for VAP development include recent surgery, use of proton pump inhibitors, prolonged endotracheal tube (ETT) placement and difficulty in weaning from mechanical ventilation (i.e., need for re-intubation), nasogastric tube placement, supine patient position, recent use of antibiotics, chronic pulmonary disease, trauma and previous septicaemia [4]. Moreover, according to some reports, transportation of the patient outside the ICU might also be associated with an increased risk of VAP [5].
The microbiological epidemiology of nosocomial pneumonias is complex. The most important pathogens causing VAP today are gram-negative bacteria (mainly Klebsiella spp, Acinetobacter spp, Pseudomonas spp, Escherichia coli and other Enterobacteriaceae) and some gram-positive species such as Staphylococcus aureus and Enterococci, followed by a small amount of anaerobic species [1, 6]. Furthermore, extensive antimicrobial resistance among bacteria isolated from critically ill patients with VAP represents a serious concern for the clinician, as it may lead to increased antibiotic drug usage, higher mortality rates and prolonged length of stay in the ICU [7, 8]. Recent studies have shown an increasing spread of multidrug-resistant gram-negative and gram-positive bacteria in ICUs across Europe and the United States in the last few years, with carbapenem resistance usually found in almost 70 % of Acinetobacter spp and 40 % of Klebsiella spp and an extended-spectrum beta lactamase (ESBL) pattern expressed in up to 30 % of Enterobacteriaceae isolates globally [9]. Generally, mechanical ventilation, immunosuppression, recent antimicrobial therapy, hospitalization longer than 5 days, chronic dialysis, and residence in a nursing home or extended care facility are considered the most relevant risk factors for acquiring a respiratory nosocomial infection caused by multidrug-resistant pathogens in the ICU [10].
This study retrospectively investigated VAP incidence in the adult ICU to evaluate the incidence rate of VAP, causative organisms, outcomes of infection and the most significant clinical predictors of VAP development, including the usual risk factors for acquiring nosocomial infections on admission to the ICU. As a secondary end point, the study aimed to identify the antimicrobial susceptibility patterns and prevalence of multi-drug resistant gram-positive and gram-negative bacteria in the respiratory specimens in all patients who were diagnosed with ventilator-associated pneumonia in the ICU.
Methods
The study was conducted as a post-hoc analysis of a prospectively collected database over a 12-month period from September 2010 to September 2011. All patients admitted into the adult ICUs of six different hospitals in Rome, Italy, were monitored by the attending physicians for development of ventilator associated pneumonia (VAP). Patients’ main demographics, risk factors, cause of admission (when available), and comorbidities were identified; cultures collected from respiratory samples were acquired, and all the outcomes were registered. Antimicrobial susceptibility testing was performed, and the most common patterns of drug resistance for gram-positive and gram-negative isolates obtained from respiratory specimens were identified.
Among the hospitals involved in the study, three were teaching hospitals (University of Tor Vergata, University of La Sapienza, and S. Andrea Hospital) and three were non-teaching hospitals (S. Giovanni Hospital, S. Gallicano Hospital, and S. Filippo Neri Hospital).
Clinical samples for microbiological culture comprised bronchoalveolar lavage (BAL) and bronchial aspirates (BAS). Cultures were processed using standard microbiological methods. Identification of isolates was performed with the VITEK (bioMérieux, Durham, NC) and API automated systems (bioMérieux, Marcy l’Etoile, France). Organism identification and susceptibility classification were completed with the Vitek 2 system (bioMérieux, Balmes-les-Grottes, France) and by manual biochemical identification when necessary. Antimicrobial susceptibility testing was performed on all isolates with the Vitek 2 system as approved by the United States Food and Drug Administration to determine MICs. Organism susceptibility was interpreted according to CLSI guidelines [11].
VAP in the ICU was defined according to ATS/IDSA guidelines [3], as a nosocomial pneumonia with an onset time beyond 48 h after the patient has been placed on mechanical ventilation with the presence of clinical and radiological signs that are suggestive of a new infective process of the lung (e.g., a new or worsening pulmonary infiltrate on chest X-ray, fever > 38 °C, elevated WBC > 12,000/mmc or leukopenia < 4,000/mmc and onset of significant bronchial purulent discharge). Early onset or late-onset VAP were defined whether the infectious process occurred within or after 96 h of ICU admission.
The study was funded by the Italian Drug Agency (AIFA) as part of a project aimed at monitoring the microbiological epidemiology of Roman ICUs. The study was approved by all the ethics committees of the centres involved.
Statistical analysis
The incidence rate (IR) of VAP was calculated as the ratio between the number of VAP cases and the total number of days in the ICU, and IRs were expressed per 1,000 patient-days in the ICU. IRs were also calculated by stratifying the cohort by several patient characteristics [i.e., sex, age at ICU entrance, main underlying comorbidities and referring admission department (grouped as clinical, surgical and emergency)] and by the most important causes for admission into the ICU (grouped according to the main organ dysfunction that was initially detected). Using mixed (by clinical centres) Poisson regression models, crude and adjusted incidence rate ratios (IRR) were calculated to evaluate the most important clinical predictors for VAP in the ICU comparative with previously described clinical predictors. Adjusted IRRs were obtained by a multiple regression model that only included covariates from the univariate analysis with a p-value <0.2.
Finally, the Kaplan-Meier method was used to estimate the cumulative probability of survival in the ICU. To evaluate if VAP was associated with a greater risk of death, an analysis was also performed that stratified patients by VAP and non-VAP occurrence. Data were allocated in a time-dependent fashion from patients in whom VAP occurred [12].
Statistical analyses were performed using the STATA statistical software package (Stata Statistical Software: Release 12, 2012. StataCorp LP, College Station, TX, USA).
Results
In the whole cohort, 115 patients (6.9 %) developed at least one episode of VAP in the ICU, with an incidence rate of 5.82 (95 % CI 4.85–6.99) cases per 1000 patient-days. Among these cases, 36 (31.3 %) were early-onset VAP and 79 (68.7 %) were late-onset VAP. Table 1 shows patient characteristics by referring department and VAP occurrence. Overall, a total of 1,647 patients—1,034 (62.7 %) males and 613 (37.3 %) females—were enrolled in the study, with a median age of 69 years (IQR 56–77). Three hundred fifty-seven (21.6 %) patients were referred to the ICU from medical wards, 651 (39.5 %) from surgical wards, 516 (31.3 %) from the emergency department, and 123 (7.4 %) patients were directly transferred from other health centres. The most common reasons for admission into the ICU in the study population were post-operative complications, pulmonary disease, CNS or neurological disease, trauma and gastrointestinal disease (Table 1). The median length of ICU stay before VAP onset was 6 days (IQR 3–11), with the highest percentages occurring in patients admitted from the emergency department (11.4 %) followed by those admitted from medical wards (3.6 %) and surgical departments (3.5 %). The median age of VAP patients was 65.1 years (IQR 50.5–76.1). Moreover, we observed a median length of stay in the ICU of 27 days (IQR 16–40) in patients with VAP versus 3 days (1–10, IQR) in those without VAP.
In the univariate analysis of risk factors for development of VAP (Table 1), the highest incidence rates (IR, episodes per person/year) of pneumonia were observed in patients with COPD (IR 6.31), heart disease (IR 7.42), diabetes (IR 6.34), obesity (IR 10.84) and renal failure (IR 8.58) and in patients admitted into the ICU due to neurological disease (IR 7.48), trauma (IR 6.2) or cardiovascular disease (IR 8.74), whereas the lowest incidence rates of VAP were observed in patients admitted due to urinary or gastrointestinal disease.
In the multivariate analysis (Table 2), admittance into the ICU for CNS disease was significantly associated with increased relative risk of VAP when compared with admission due to GI disease (p =0.02, aIRR 4.06, 1.24–13.68 95 % CI) and emergency referral from other hospitals was associated with a higher risk of disease when compared with referral from medical wards (p = 0.04, aIRR 2.11, 1.03-4.31 95 % CI). Moreover, although not reaching statistical significance, increased relative risk for VAP was observed in patients admitted for trauma, cardiovascular disease or respiratory disease and for patients with obesity or renal failure.
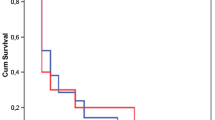
The overall mortality rate in the study for all causes was 25 % (412 of 1,647 patients). Out of 115 patients with VAP, a total of 51 patients died, with a case fatality rate of 44.3 %. Kaplan-Meier survival analysis of the whole patient cohort showed a median ICU survival of 30 days (95 % CI 25–34) (Fig. 1a). A time-dependent survival analysis in which the risk of VAP was corrected by the length of stay showed a slightly higher risk of death in the non-VAP group versus the VAP group in the first days after the admission, with a subsequent reversal of this trend in the final days of ICU stay. However, this difference was not statistically significant (p = 0.60, Fig. 1b). Moreover, we observed a median survival of 34 days (95 % CI 25–52) since VAP onset among the VAP subgroup (data not shown). After stratifying the VAP group by early- or late-onset episodes, we observed a slightly higher survival probability in patients diagnosed with early-onset compared with those with late-onset VAP (p = 0.17, data not shown).
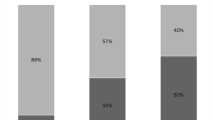
A total of 372 isolates were collected from bronchial aspirates and bronchoalveolar lavages of ICU patients who were diagnosed with VAP (Table 3). Microbiological analysis of the respiratory tract pathogens showed that 68 % (n = 253) were gram-negative organisms, 24.7 % (n = 92) were gram-positive organisms, and 7.3 % (n = 27) were Candida spp. In the gram-positive cocci group, Coagulase negative staphylococci (CoNS) were the most prevalent (40.2 %), followed by Staphylococcus aureus (34.8 %), Enterococcus spp (15.2 %), and other gram-positive species. Among the most relevant gram-positive cocci, resistance rate to oxacillin (MRSA) was detected in 56 % of S. aureus isolates, whereas no resistance to ampicillin or to high concentrations of gentamicin was detected in Enterococcus species (data not shown). In the gram-negative bacilli group, Klebsiella pneumoniae was the most common species (28.8 %), followed by Acinetobacter baumannii (26.9 %), Pseudomonas aeruginosa (20.5 %), Escherichia coli (7.5 %), and Proteus mirabilis (5.9 %). Antimicrobial susceptibility patterns (Table 4) among the gram-negative group showed resistance to amikacin in 64 %, 44 % and 22.7 % of Klebsiella pneumoniae, Acinetobacter spp, and Pseudomonas aeruginosa isolates, respectively. Ceftazidime susceptibility was retained in approximately 55 % of Pseudomonas strains. Resistance to meropenem was identified in 74 %, 76 % and 33 % of K. pneumoniae, Acinetobacter spp, and P. aeruginosa isolates, respectively. Overall, 29 % of K. pneumoniae isolates and 6 % of A. baumannii isolates showed resistance to colistin, while susceptibility to tigecycline was retained in 100 % and 95 % of isolates. Moreover, resistance to fluoroquinolones was detected in approximately 76 % of Klebsiella spp, 97 % of Acinetobacter spp, 33 % of P. aeruginosa strains, and 29 % of E. coli strains.
Discussion
Among the nosocomial infections, ventilator-associated pneumonia acquired in the intensive care unit (ICU) represents a significant and dreadful complication worldwide that is often associated with highly resistant pathogens in the respiratory tract [13, 14]. In our work, we found an incidence of VAP of 5.82 cases per 1,000 patient-days (almost 7 % of all ICU population), which is consistent with other ECDC reports [15]. In particular, this is a slightly lower rate when compared to the rate of 8.0 cases per 1,000 ventilator-days reported in the German Krankenhaus Infektions Surveillance System (KISS) and to a median rate of 16.4 cases per 1,000 ventilator-days in the French national surveillance system [16, 17]. This difference might partly be explained by the fact that in our research we registered VAP incidence as number of cases per patient-days in ICU, since this might apparently underestimate the incidence of the infectious episodes to some extent, as described in previous reports [18]. However, all of the European cohorts described above seem to have a significantly higher incidence of VAP in comparison with the rate of 1.9–2.9 cases per 1000 ventilator-days in medical-surgical ICUs (median rate, 1.3–2.0 cases per 1000 ventilator-days) reported in the North American National Healthcare Safety Network (NHSN) [19]. As stated by other authors, several factors may contribute to these differences in estimating VAP incidence in the ICU in diverse countries. In fact, criteria for defining VAP may differ across networks, and a vast number of medical conditions occurring in the ICU such as atelectasis, pulmonary edema, acute respiratory distress syndrome, hypersensitivity pneumonitis, or pulmonary hemorrhage are reported to mimic pneumonia, and they all may represent confounding factors for VAP diagnosis [20, 21]. Finally, in our study we found a proportion of early-onset VAP up to 31 %, with the rest of the cases represented by late-onset (>96 h) episodes; this data is aligned with similar reports in literature [22].
In our statistical analysis, a higher risk of VAP was observed in patients who were admitted to the ICU from emergency departments or who were referred to medical centres for treatment of urgent diseases from other hospitals. This phenomenon may be explained by factors including urgent intubation (e.g., trauma or cardiorespiratory arrest) and patient transportation outside the ICU, which have previously been reported to facilitate the infective process and may account for a lack of preventive measures during intubation manoeuvres [23–25]. Moreover, in accordance with other research [26–28], we found an increased incidence rate of VAP in patients admitted to the ICU for neurological complications (including stroke, post-traumatic and metabolic coma) and in trauma patients. Finally, although not statistically significant, we found higher VAP rates in obese patients and in patients with a history of chronic renal failure.
According to other studies, an episode of VAP in the ICU is significantly associated with higher rates of in-hospital mortality [29, 30]. In our study, developing VAP in the ICU was associated with a 19 % increase in the risk of in-hospital mortality (from 25 to 44 %), which is similar to the findings of other works [1, 31, 32]. Next, in our time-dependent survival analysis, we could detect a slightly higher risk of death in the non-VAP group versus the VAP group in the first days after the admission, with a subsequent reversal of this trend in the final days of ICU stay. These findings can be explained by the fact that a higher mortality rate was generally observed during the very first days of an ICU stay in all patients (presumably due to presenting with the most critical conditions) before sufficient time had elapsed to develop VAP. On the other hand, patients who survive longer have a greater risk of developing VAP in the future. A higher mortality rate was observed in this subgroup towards the end of the survival curve as time since admission into the ICU increased. However, the difference in mortality between the two groups was not statistically significant. This fact might be related to the limited number of patients enrolled (i.e. to lacking of statistical potency of the sample), and should be better verified in larger prospective cohorts.
In relation to our microbiological analysis, our study found an extremely high incidence of resistance to carbapenems among Klebsiella pneumoniae strains isolated from respiratory specimens, which is consistent with the recent spread of KPC K. pneumoniae subtypes across south European countries in recent years [33, 34]. In the European Antimicrobial Resistance Surveillance Network (EARS-Net) surveillance system, this phenomenon has been reported to remain stable until 2009 in most countries except Greece and Italy, where an increase has been registered from 1–2 % in 2006–2009 to 15.2 % in 2010, and up to 27 % in 2011 [35, 36]. Moreover, recent surveys conducted by Italian local surveillance systems have revealed a persistent condition of KPC-Kp endemicity in different hospitals in North Italy [37, 38]. In general, poor functional status, ICU stay, transplantation, mechanical ventilation, prolonged hospital stay and previous antimicrobial treatment are all recognized as major risks factors for nosocomial acquisition of KPC K. pneumoniae [39]. In this context, we think that clinicians should pay special attention to the emergence of carbapenem-resistant strains among gram-negative bacteria in the ICU, since infections due to these species are notably associated with higher mortality rates and therapeutic failure, often requiring complex and expensive antibiotic treatments [40, 41]. Furthermore, in our microbiological analysis we were able to detect Candida species in almost 7.3 % of cases (27 samples). In our work, Candida spp. isolation in respiratory specimens was not associated with the simultaneous presence of candidemia, therefore, it was more likely to be considered as a colonization. However, this data should be interpreted cautiously, since according to recent works, Candida spp. isolation in respiratory samples in ICU patients affected by nosocomial pneumonia seems to be associated with more initial disease severity without influencing the final outcome of these patients, and the role of the antifungal therapy against these isolates in VAP patients has to be clarified yet [42, 43]. On the other hand, Candida spp. airway colonization in mechanically ventilated ICU patients has recently been reported to be associated with subsequent Acinetobacter baumannii VAP, so that this phenomenon might deserve some attention as it seems to have an influence on developing further microbial colonization and infection in the respiratory tract [44].
Some limitations to our study should be mentioned. Given the retrospective nature of our analysis, we were unable to obtain reliable data on the specific cause of death in each patient of our cohort. Moreover, antimicrobial resistance patterns were only phenotypically identified because a genotypic assay for ESBL and KPC resistance genes was not available in all the centres involved. Next, we did not manage to furnish a time-dependent evaluation of the microbial species in the respiratory specimens in our cohort, which would be useful to better understand the changing ecological pattern over the length of stay in the ICU. Finally, we could detect a tendency towards an increased risk of VAP in obese patients and in subjects who suffered from chronic renal failure, but this data needs to be corroborated by larger observational cohorts to increase the statistic potency of our analysis.
However, our study showed that identification of the cause for admission into the ICU may be of interest in predicting VAP development, especially for patients suffering from CNS disease, trauma or cardiovascular disease and for patients with emergency referral into the ICU. At the same time, the emergence of high rates of antimicrobial resistance particularly among gram-negative species in the ICU in recent years might represent a serious concern in addressing these events in the near future. In conclusion, both clinical evaluation on admission and accurate microbiologic sampling of the respiratory tract are key to understanding the complex management of these kinds of nosocomial infections, and further prospective investigations are needed to fully understand the clinical determinants of VAP development in the ICU environment.
References
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Wilke M, Grube R (2014) Update on management options in the treatment of nosocomial and ventilator assisted pneumonia: review of actual guidelines and economic aspects of therapy. Infect Drug Resist 7:1–7
American Thoracic Society; Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. ATS/IDSA. Am J Respir Crit Care Med 171:388–416
Rea-Neto A, Youssef NC, Tuche F, Brunkhorst F, Ranieri VM, Reinhart K et al (2008) Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care 12:R56
Kollef MH, Von Harz B, Prentice D, Shapiro SD, Silver P, St John R et al (1997) Patient transport from intensive care increases the risk of developing ventilator-associated pneumonia. Chest 112:765–773
Sandiumenge A, Rello J (2012) Ventilator-associated pneumonia caused by ESKAPE organisms: cause, clinical features, and management. Curr Opin Pulm Med 18:187–193
Piskin N, Aydemir H, Oztoprak N, Akduman D, Comert F, Kokturk F et al (2012) Inadequate treatment of ventilator-associated and hospital-acquired pneumonia: risk factors and impact on outcomes. BMC Infect Dis 12:268. doi:10.1186/1471-2334-12-268
Martin-Loeches I, Torres A, Rinaudo M, Terraneo S, de Rosa F, Ramirez P et al (2015) Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J Infect 70:213–222
Wroblewska MM, Rudnicka J, Marchel H, Luczak M (2006) Multidrug-resistant bacteria isolated from patients hospitalised in intensive care units. Int J Antimicrob Agents 27:285–289
Denys GA, Relich RF (2014) Antibiotic resistance in nosocomial respiratory infections. Clin Lab Med 34:257–270
CLSI (2010) Performance standard for antimicrobial susceptibily testing; twenty-first information supplement. CLSI M100, S20
Kalbfleisch JD, Prentice RL (2002) The statistical analysis of failure-time data, 2nd edn. Wiley, New York
Medell M, Hart M, Duquesne A, Espinosa F, Valdés R (2013) Nosocomial ventilator-associated pneumonia in Cuban intensive care units: bacterial species and antibiotic resistance. MEDICC Rev 15:26–29
Quartin AA, Scerpella EG, Puttagunta S, Kett DH (2013) A comparison of microbiology and demographics among patients with healthcare-associated, hospital-acquired, and ventilator-associated pneumonia: a retrospective analysis of 1184 patients from a large, international study. BMC Infect Dis 13:561. doi:10.1186/1471-2334-13-561
European Centre for Disease Prevention and Control (2012) Surveillance of health-care associated infections in Europe 2007. Report. Stockholm: ECDC; 2012. http://ecdc.europa.eu/en/publications/Publications/120215_SUR_HAI_2007.pdf
Gastmeier P, Geffers C, Brandt C, Zuschneid I, Sohr D, Schwab F et al (2006) Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect 64:16–22
INVS (2007) Réseau d’alerte d’investigation et de surveillance des infections nosocomiales (RAISIN). Surveillance des infections nosocomiales en réanimation adulte. France, résultats 2006, 2007. www.invs.sante.fr/content/download/.../rea_raisin_resultats_2007.pdf.
Uçkay I, Ahmed QA, Sax H, Pittet D (2008) Ventilator-associated pneumonia as a quality indicator for patient safety? Clin Infect Dis 46:557–563
Edwards JR, Peterson KD, Andrus ML, Dudeck MA, Pollock DA, Horan TC (2008) National Healthcare Safety Network (NHSN) report, data summary for 2006 through 2007, issued November 2008. Am J Infect Control 36:609–626
Bouadma L, Deslandes E, Lolom I, Le Corre B, Mourvillier B, Regnier B et al (2010) Long-term impact of a multifaceted prevention program on ventilator-associated pneumonia in a medical intensive care unit. Clin Infect Dis 51:1115–1122
Klompas M, Kulldorff M, Platt R (2008) Risk of misleading ventilator-associated pneumonia rates with use of standard clinical and microbiological criteria. Clin Infect Dis 46:1443–1446
Gadani H, Vyas A, Kar AK (2010) A study of ventilator-associated pneumonia: Incidence, outcome, risk factors and measures to be taken for prevention. India J Anaesth 54:535–540
Ranjan N, Chaudhary U, Chaudhry D, Ranjan KP (2014) Ventilator-associated pneumonia in a tertiary care intensive care unit: analysis of incidence, risk factors and mortality. India J Crit Care Med 18:200–204
Hui X, Haider AH, Hashmi ZG, Rushing AP, Dhiman N, Scott VK et al (2013) Increased risk of pneumonia among ventilated patients with traumatic brain injury: every day counts! J Surg Res 184:438–443
Decelle L, Thys F, Zech F, Verschuren F (2013) Ventilation-associated pneumonia after intubation in the prehospital or the emergency unit. Eur J Emerg Med 20:61–63
Jovanovic B, Milan Z, Markovic-Denic L, Djuric O, Radinovic K, Doklestic K et al (2015) Risk factors for ventilator-associated pneumonia in patients with severe traumatic brain injury in a Serbian trauma centre. Int J Infect Dis 38:46–51
Plurad DS, Kim D, Bricker S, Lemesurier L, Neville A, Bongard F et al (2013) Ventilator-associated pneumonia in severe traumatic brain injury: the clinical significance of admission chest computed tomography findings. J Surg Res 183:371–376
Gianakis A, McNett M, Belle J, Moran C, Grimm D (2015) Risk factors for ventilator-associated pneumonia: among trauma patients with and without brain injury. J Trauma Nurs 22:125–131
Zhu S, Cai L, Ma C, Zeng H, Guo H, Mao X et al (2015) The clinical impact of ventilator-associated events: a prospective multi-center surveillance study. Infect Control Hosp Epidemiol 27:1–8
Boyer AF, Schoenberg N, Babcock H, McMullen KM, Micek ST, Kollef MH (2015) A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest 147:68–81
Safdar N, Dezfulian C, Collard HR, Saint S (2005) Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 33:2184–2193
Efrati S, Deutsch I, Antonelli M, Hockey PM, Rozenblum R, Gurman GM (2010) Ventilator-associated pneumonia: current status and future recommendations. J Clin Monit Comput 24:161–168
Djahmi N, Dunyach-Remy C, Pantel A, Dekhil M, Sotto A, Lavigne JP (2014) Epidemiology of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Mediterranean countries. Biomed Res Int 2014:305784
Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M et al (2012) Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431
European Centre for Disease Prevention and Control (ECDC) (2012) Antimicrobial resistance surveillance in Europe 2011. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). ECDC, Stockholm
Corcione S, Rocchetti A, Argentero PA, Raso R, Zotti CM, De Rosa FG et al (2015) A one-year survey of carbapenemase-producing Klebsiella pneumoniae in Italy: beyond the ICU. Clin Microbiol Infect 21:e11–e13
Corbellini S, Caccuri F, Gelmi M, De Francesco MA, Fiorentini S, Caruso A et al (2014) Emergence of carbapenem-resistant Klebsiella Pneumoniae strains producing KPC-3 in Brescia Hospital, Italy. New Microbiol 37:177–183
Parisi SG, Bartolini A, Santacatterina E, Castellani E, Ghirardo R, Berto A et al (2015) Prevalence of Klebsiella pneumoniae strains producing carbapenemases and increase of resistance to colistin in an Italian teaching hospital from January 2012 To December 2014. BMC Infect Dis 15:244
Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R (2009) Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol 30:666–671
Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, Canadian Critical Care Trials Group et al (2008) Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care 23:18–26
Chittawatanarat K, Jaipakdee W, Chotirosniramit N, Chandacham K, Jirapongcharoenlap T (2014) Microbiology, resistance patterns, and risk factors of mortality in ventilator-associated bacterial pneumonia in a Northern Thai tertiary-care university based general surgical intensive care unit. Infect Drug Resist 7:203–210
Terraneo S, Ferrer M, Martín-Loeches I, Esperatti M, Di Pasquale M, Giunta V et al (2016) Impact of Candida spp. isolation in the respiratory tract in patients with intensive care unit-acquired pneumonia. Clin Microbiol Infect 22:94.e1-8
Khorvash F, Abbasi S, Yaran M, Abdi F, Ataei B, Fereidooni F et al (2014) Molecular detection of Candida spp. and Aspergillus fumigatus in bronchoalveolar lavage fluid of patients with ventilator-associated pneumonia. J Res Med Sci 19:S46–S50
Tan X, Zhu S, Yan D, Chen W, Chen R, Zou J et al (2016) Candida spp. airway colonization: a potential risk factor for Acinetobacter baumannii ventilator-associated pneumonia. Med Mycol. 2016 Mar 21. pii: myw009. [Epub ahead of print]
Acknowledgments
This study was partially presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC abstract K-332], San Diego, CA, USA, September 10–13, 2015.
Authors’ contributions
DDR and PP contributed to the design of the study and wrote the manuscript. GPT, MA, LS and PS designed the study and coordinated field sampling. LS and MA reviewed the manuscript. SG and SB provided support in the study design and supplied the software for data collection. MM, MTG, GP, RC, MDA, GC, MR, FL, SN, CF, MF, MGC, and TF performed data collection at the hospitals in this study and completed microbiological analysis. EF contributed to the final manuscript. PP performed and interpreted the statistical analysis and reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was financially supported by a grant from the Italian Drug Agency (AIFA), Pharmacovigilance Projects in the Lazio Region, Analysis of Nosocomial Infections in Roman Intensive Care Units, 2009. Grant N° D 3012 28/09/2009.
Conflict of interest
None declared.
Ethical approval
The study was approved by the ethics committees of all of the centres involved.
Informed consent
Because of the retrospective nature of the study, informed consent was not required.
Additional information
G.P. Testore died before the conclusion of the study.
Rights and permissions
About this article
Cite this article
Delle Rose, D., Pezzotti, P., Fortunato, E. et al. Clinical predictors and microbiology of ventilator-associated pneumonia in the intensive care unit: a retrospective analysis in six Italian hospitals. Eur J Clin Microbiol Infect Dis 35, 1531–1539 (2016). https://doi.org/10.1007/s10096-016-2694-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2694-9