Abstract
Carbapenem-resistant Klebsiella pneumoniae (CRKP) has emerged during recent years in several intensive care units. The objective of our study was to determine the incidence of CRKP and the risk factors associated with acquisition during intensive care unit (ICU) stay. This prospective cohort study was conducted between May 2007 and April 2008 in a medical-surgical ICU at a tertiary medical center. Rectal surveillance cultures were obtained from patients on admission and twice weekly. Of screened patients, 7.0% (21/299) were CRKP colonized on admission to the ICU. One hundred eighty (81%) patients were screened at least twice. Of these, 48 (27%) patients acquired CRKP during ICU stay. Of the 69 CRKP colonized patients (both imported and ICU acquired), 29% (20/69) were first identified by microbiologic cultures, while screening cultures identified 49 patients (71%). Of these, 23 (47%) subsequently developed clinical microbiological cultures. Independent risk factors for CRKP acquisition included recent surgery (OR 7.74; CI 3.42–17.45) and SOFA score on admission (OR 1.17; CI 1–1.22). In conclusion, active surveillance cultures detected a sizable proportion of CRKP colonized patients that were not identified by clinical cultures. Recent surgical procedures and patient severity were independently associated with CRKP acquisition
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acquisition of multidrug resistant microorganisms (MDRO) is a major threat for hospitalized patients in intensive care units (ICUs). Possible sources for acquisition of MDRO include other colonized patients, health care workers and the ICU environment. Many variables may increase the risk of MDRO acquisition, such as multiple comorbidities, invasive procedures, presence of indwelling devices, close proximity to a colonized patient, high colonization pressure in the unit and antibiotic exposure [1–5].
Since the first description of carbapenem-resistant Klebsiella pneumoniae (CRKP) in North Carolina in 2001 [6], several outbreaks have been reported worldwide [7–9]. In a report from New York, CRKP accounted for one-quarter of all invasive Klebsiella pneumoniae infections [10]. During recent years, CRKP has spread in several health care facilities in Israel [11–15]. Recent molecular studies have found that closely related K. pneumoniae strains carrying bla KPC- have caused outbreaks in the United States and Israel [12, 16]. The occurrence of a dominant clone in two continents demonstrates the potential of a single extensively drug-resistant strain to spread globally. It was suggested that the acquisition of a bla KPC gene may confer on this isolate of K. pneumoniae a marked selective advantage that is leading to its successful dissemination [17]. An alternative hypothesis is that the strain has some selective advantage (e.g., for dissemination or for colonization) and blaKPC adds to this advantage by making the strain more resistant. The high mortality rates associated with CRKP nosocomial infections [10, 18, 19], has led national organizations to develop intensified strategies to limit further spread in healthcare facilities [20]. Further understanding of the dynamics of transmission and risk factors of CRKP acquisition is essential to designing effective control measures.
During 2006, an epidemic clone of CRKP spread in Sheba Medical Center. The number of CRKP clinical cases increased gradually from 3–5 cases each month (1.08/10,000 patient-days) during the first quarter of 2006 to 22–24 cases each month (6.93/10,000 patient-days) during the last quarter of 2007 [15]. Twenty percent of patients have acquired CRKP in intensive care units. Pulsed-field gel electrophoresis analysis demonstrated the spread of a single clone in the facility. PCR revealed the presence of bla KPC-3 in all isolates. In May 2007, we initiated a hospital-wide intervention program, leading to a significant decrease in nosocomial CRKP acquisition. Of note, the decrease in acquisition was observed after a period of several months. During the first period of the intervention we noticed high rates of acquisition in a medical–surgical ICU. Previous studies have reported that ICU stay and prior antibiotic exposure were associated with CRKP acquisition [13, 18]. However, no studies have assessed risk factors for CRKP acquisition among ICU patients. We therefore undertook this study to evaluate baseline colonization rates on admission, cross-transmission rates within an ICU and risk factors associated with ICU acquisition.
Methods
Setting
Sheba Medical Center is a 1,600-bed tertiary referral hospital. It is a major center for trauma, neurosurgery, bone-marrow transplantations and rehabilitation. The 11-bed ICU is both a medical ICU and a surgical ICU with a large numbers of trauma patients. The unit is designed as an open ward. The nurse–patient ratio is 1:2.
Study design and population
We conducted a prospective cohort study of patients admitted to the ICU from May 2007 through April 2008. We obtained rectal swab cultures within 72 hours of ICU admission and twice weekly while the patient was in the ICU, to detect CRKP colonization. Patients who were colonized with CRKP prior to admission or with positive cultures during the first 72 hours were defined as imported CRKP. Patients with negative culture results on the time of admission, who acquired CRKP in subsequent cultures, were defined as acquired CRKP. Daily colonization pressure was calculated as the daily number of patients colonized with CRKP divided by the daily number of patients treated in the ICU. Location of the patients in the unit was recorded and proximity to another CRKP colonized patient was defined as hospitalization in an adjacent bed.
Data collected for all study patients included demographic characteristics, date of hospital and ICU admission, Charlson comorbidity score [21] and antibiotic use. For colonized patients, antibiotic use was collected up until the point of CRKP detection. Acute physiology and chronic health evaluation II (APACHE score) [22], and the sequential organ failure assessment (SOFA) score [23] were calculated prospectively for all patients on admission to the unit. Nosocomial infections were defined according to the Centers for Disease Control and Prevention National Nosocomial Infections Surveillance System [24].
Infection control measures
During the study period, from May 2007 through April 2008, colonized patients were cohorted in a designated area of the unit, and contact precautions were used by staff providing care to the patients.
Microbiology methods
Surveillance cultures were collected on a Copan Amies sterile transport swab (Copan Diagnostics, Corona, CA) and transported to the microbiology laboratory. Rectal swabs were streaked onto MacConkey agar plate (Hy-Lab, Rehovot, Israel) with meropenem (10μg) and ertapenem (10μg) disks and were incubated overnight at 35°C in ambient air. Bacterial colonies in the area surrounding either disk were isolated and identified by standard laboratory methods following Clinical and Laboratory Standards Institute guidelines [25]. The swabs were also cultured in brain–heart infusion broth, initially with a disk of ertapenem and thereafter without any selective substances. After overnight incubation, a subculture of all the broths was made onto MacConkey agar plates (Hy-Lab, Rehovot, Israel). All three carbapenem disks (ertapenem [10μg], meropenem [10μg], and imipenem [10μg]; Oxoid, UK) were placed on the plates and incubated for another 18–24 hours. Bacteria growing in the colonies were identified using routine methods [25]. All isolates were screened for bla KPC by PCR as we described previously [26]. We developed and validated a real-time PCR assay for detecting bla KPC genes. Briefly, bacterial DNA was extracted from vortexed perianal swabs. TaqMan technology was used for detecting bla KPC , using primer sequences that were designed in-house. Sensitivity and specificity of the real-time PCR was 100% and 95%, respectively [26].
Statistical methods
T-test and χ2 test were used for comparisons. Logistic regression was performed to assess factors independently associated with ICU CRKP acquisition. All variables associated with CRKP colonization in the univariate analysis (p <0.2) were included as candidate variables in the multivariable analysis. A stepwise model was used. Variables were included in the final model if they were significant at p < 0.05. Analyses were performed using Stata software (Stata Corp., College Station, TX).
Approval for the study was obtained from the local Research Ethics Committee.
Results
During the study period, 365 patients were admitted to the ICU. A total of 299 (82%) patients were screened within 72 hours. Of the 278 initially CRKP negative, 223 (80%) patients had an ICU stay of more than 72 hours. Compliance with obtaining at least two cultures was 81% (180/223).
Imported CRKP
CRKP was isolated on admission from 21 (7%) of 299 patients. Of these, 5 (24%) patients were previously known to be colonized. The average length of stay before admission to the unit of colonized patients and non-colonized patients was 15.3 (SD ±18.4) and 7.4 (SD ±13.4) (p < 0.001), respectively.
Acquisition in the unit
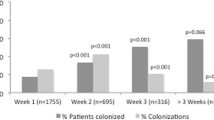
Forty-eight patients (27%) of the 180 initially CRKP negative patients acquired CRKP during ICU stay, after a mean of 9.6 (SD ± 5.4) days. Of these, 33 (68.8%) were identified first by screening cultures, while clinical cultures identified 15 patients (31.2%). Fifty percent of acquisitions were detected within the first week of stay in the unit. During the study period, there were 24 weeks without acquisitions, 21 weeks with one acquisition, 5 with two acquisitions, 3 weeks with three and 2 weeks with four acquisitions. The average daily colonization pressure was 32% (SD 17.5%; range 0–71.4%).
Of the 69 CRKP colonized patients (both imported and ICU acquired), 29% (20/69) were first identified by microbiologic cultures, while screening cultures identified 49 patients (71%). Of these, 47% (23/49) of patients subsequently developed clinical microbiological cultures. The median duration between screening cultures and the recovery of clinical microbiologic cultures was 13.6 days (range 2–66 days).
Risk factors for CRKP acquisition during ICU stay
Patients’ demographic and comorbidities characteristics are shown in Table 1. With respect to age, gender, or underlying comorbidities, we observed no significant differences between CRKP colonized and non-colonized groups. There was a trend towards worse Charlson comorbidity scores in patients who acquired CRKP during ICU stay (p = 0.06). Events that occurred after hospitalization are described in Table 2. Patients who acquired CRKP had a higher SOFA score (p = 0.05) and were more likely to undergo surgery during hospitalization (p < 0.001). A higher colonization pressure in the unit was measured on the day of admission among those who acquired CRKP colonization (p = 0.049). There was no difference in prior antibiotic exposure or specific antibiotic exposure. Variables included in the multivariate logistic regression were myocardial infarction, peptic ulcer, Charlson comorbidity score, SOFA score, prior antibiotic use, colonization pressure and recent surgery. Recent surgery (OR 7.74; CI 3.42–17.45) and SOFA score on admission (OR 1.17; CI 1–1.22) were independent risk factors for CRKP acquisition.
Outcome
Twenty of 48 (42%) patients who acquired CRKP during ICU stay developed nosocomial infection with CRKP: nine catheter associated bloodstream infection, four surgical-site infections and seven patients developed ventilator associated pneumonia. Patients who acquired CRKP had a longer length of stay in the unit. There was no difference both in ICU mortality rates and in-hospital mortality rates (Table 3).
Discussion
The rapid and extensive spread of KPC-producing Enterobacteriacea in several healthcare facilities worldwide is an alarming phenomenon [27]. While a number of studies have suggested that hospital acquisition of KPC-harboring isolates is associated with antibiotic exposure and prolonged length of stay [13, 18], little is known on the dynamics of CRKP acquisition and transmission in ICUs. We observed high acquisition rates in a medical-surgical ICU, with more than a quarter of the patients admitted to the ICU having acquired CRKP during the study period. Most of the acquisitions occurred within the first week. Recent surgical procedures and high SOFA score were independently associated with acquisition. A high proportion of patients who acquired CRKP subsequently developed a nosocomial infection during ICU stay.
We found that active surveillance has identified a large unrecognized reservoir of CRKP carriers among the ICU patients. More than 70% of colonized patients were first identified by surveillance cultures. Similar to our results, recent reports have shown that clinical cultures may fail to identify patients colonized with CRKP [10, 28]. Calfee et al. recently reported that 37% of patients with CRKP were first identified by surveillance cultures [29]. Our results suggest that active surveillance may be an essential part of preventing the spread of CRKP in the setting of outbreaks or endemic healthcare facilities. Hospitals that currently have a low prevalence of CRKP should be aggressive with active surveillance from the first clinical case and implement rapid control measures.
Other studies have assessed risk factors of CRKP infections among hospitalized patients. Antibiotic exposure, prolonged length of stay and ICU stay were found to be independent risk factors [13, 18]. However, most studies described patients with clinical isolates. Therefore, misclassification of patients identified as control groups may occur. Recently, a point-prevalence survey conducted in a single medical center found a carriage rate of 5.4% among 298 hospitalized patients. Risk factors for colonization were prolonged length of stay, vancomycin exposure and diaper use. In contrast to other studies, we conducted a prospective cohort study, focusing on risk factors for CRKP acquisition during ICU stay. In addition to individual risk factors, we also examined ecological risk factors including colonization pressures and proximity to other colonized patients. In contrast to the results of prior studies, CRKP acquisition was not associated with prior antibiotic therapy. This finding may be related to the high rate of antibiotic exposure during ICU stay among the total study population (>95%). Thus, it was not possible to assess the impact of antibiotic exposure.
High colonization pressure was associated with the spread of antibiotic-resistant bacteria in several studies [1–3]. A study in a French medical ICU demonstrated that the weekly colonization pressure was the only independent predictor for nosocomial methicillin resistant Staphylococcus aureus acquisition [3]. Similarly, a study of vanomycin resistant Enterococci in a medical ICU concluded that colonization pressure was the most important variable affecting acquisition [2]. The impact of colonization pressure on the risk of resistant Gram negative bacteria acquisition was not studied. In the present study, colonization pressure was not found to be independently associated with CRKP acquisition. However, on average, more than one third of patients in the unit were colonized and low colonization rates were infrequent throughout the study period. Only during 25 days (7% of the study period), there were no CRKP colonized patients in the unit. The significant reservoir that was present throughout the study period, as well as the open design of the unit and high antibiotic pressure, may explain the high acquisition rates [30].
Severity scores were used in several studies to predict the risk of nosocomial acquisition of resistant microorganisms [31–33]. Although aggregate comorbidity measures are useful in epidemiologic research [21, 34], none of the existing measures were developed for the assessment of the risk of resistant pathogens acquisition. A recent study demonstrated that both chronic disease score and the Charlson comorbidity index were poor predictors of the risk of nosocomial infections with methicllin resistant Staphylococcus aureus or vancomycin resistant Enteroccocci [35]. We found that a high SOFA score was associated with increased risk for CRKP acquisition. Similarly, acute severity scores have performed better than Charlson comorbidity score in prediction of in-hospital mortality [36], and were associated with increased risk of resistant pathogens among ICU patients [37].
Prior surgery was found to be an independent risk factor for CRKP acquisition. There is a possibility that the acquisition occurred in the operation theatre. However, during the study period there was a low prevalence of CRKP infections in the surgical wards and it is unlikely that acquisition occurred there. The association of CRKP acquisition with recent surgery and high SOFA score may imply complexity of the patients with multiple contacts with healthcare workers.
Our study has several potential limitations. First, only 80% of patients were screened. Second, as with any surveillance screening tests, colonization may not be detected by a single swab due to intermittent colonization or lack of sensitivity of the test performed. We found that more than 50% of ICU acquisitions have occurred within the first week of stay in the ICU. Although this may truly represent acquisition of CRKP within the unit, another potential explanation is that these early cases actually represent conversion from culture negative to culture-positive due to receipt of broad spectrum antibiotics that selected for CRKP among colonized persons with low organism burdens that were undetectable at the time of ICU admission. Third, we assessed several risk factors including comorbidities, severity scores on admission to the unit, daily prevalence of CRKP, proximity to other colonized patients and antibiotic exposure. However, we did not have data on the average number of contacts with healthcare workers or compliance with infection control procedures.
To conclude, performance of active surveillance cultures has detected a large proportion of CRKP colonized patients who would otherwise not have been identified. Acquisition was associated with acuity of care and processes of care, including SOFA score and recent surgery. This important baseline data may facilitate future interventions necessary to limit CRKP transmission in this ICU.
References
Williams VR, Callery S, Vearncombe M, Simor AE (2009) The role of colonization pressure in nosocomial transmission of methicillin-resistant Staphylococcus aureus. Am J Infect Control 37:106–110
Bonten MJ, Slaughter S, Ambergen AW, Hayden MK, van Voorhis J, Nathan C et al (1998) The role of "colonization pressure" in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med 158:1127–1132
Merrer J, Santoli F, Appere de Vecchi C, Tran B, De Jonghe B, Outin H (2000) "Colonization pressure" and risk of acquisition of methicillin-resistant Staphylococcus aureus in a medical intensive care unit. Infect Control Hosp Epidemiol 21:718–723
Tacconelli E (2009) Antimicrobial use: risk driver of multidrug resistant microorganisms in healthcare settings. Curr Opin Infect Dis 22:352–358
Harris AD, McGregor JC, Johnson JA, Strauss SM, Moore AC, Standiford HC et al (2007) Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis 13:1144–1149
Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD et al (2001) Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161
Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D et al (2004) Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin Infect Dis 39:55–60
Bratu S, Landman D, Haag R, Recco R, Eramo A, Alam M et al (2005) Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med 165:1430–1435
Maltezou HC, Giakkoupi P, Maragos A, Bolikas M, Raftopoulos V, Papahatzaki H et al (2009) Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece). J Infect 58:213–219
Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP (2008) Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29:1099–1106
Leavitt A, Navon-Venezia S, Chmelnitsky I, Schwaber MJ, Carmeli Y (2007) Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob Agents Chemother 51:3026–3029
Navon-Venezia S, Leavitt A, Schwaber MJ, Rasheed JK, Srinivasan A, Patel JB et al (2009) First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 53:818–820
Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R (2009) Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol 30:666–671
Samra Z, Ofir O, Lishtzinsky Y, Madar-Shapiro L, Bishara J (2007) Outbreak of carbapenem-resistant Klebsiella pneumoniae producing KPC-3 in a tertiary medical centre in Israel. Int J Antimicrob Agents 30:525–529
Ben-David D, Maor Y, Keller N, Regev-Yochay G, Tal I, Shachar D et al (2010) Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect Control Hosp Epidemiol 31:620–626
Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, Carmeli Y et al (2009) Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 53:3365–3370
Endimiani A, Hujer AM, Perez F, Bethel CR, Hujer KM, Kroeger J et al (2009) Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 63:427–437
Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y (2008) Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 52:1028–1033
Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R et al (2009) Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 30:972–976
Centers for Disease Control and Prevention (CDC) (2009) Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. Morb Mortal Wkly Rep 58:256–260
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H et al (1996) The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332
CLSI (2006) Performance standards for antimicrobial susceptibility testing. Sixteenth informational supplement (approved standard M100-S16). The Clinical Laboratory Standards Institute, Wayne, PA
Hindiyeh M, Smollen G, Grossman Z, Ram D, Davidson Y, Mileguir F et al (2008) Rapid detection of blaKPC carbapenemase genes by real-time PCR. J Clin Microbiol 46:2879–2883
Schwaber MJ, Carmeli Y (2008) Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911–2913
Kochar S, Sheard T, Sharma R, Hui A, Tolentino E, Allen G et al (2009) Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 30:447–452
Calfee D, Jenkins SG (2008) Use of active surveillance cultures to detect asymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care unit patients. Infect Control Hosp Epidemiol 29:966–968
Pavlov I (2007) High colonization pressure might compromise the efficiency of routine methicillin-resistant Staphylococcus aureus screening. Clin Infect Dis 44:766–767
Manzur A, Gavalda L, Ruiz de Gopegui E, Mariscal D, Dominguez MA, Perez JL et al (2008) Prevalence of methicillin-resistant Staphylococcus aureus and factors associated with colonization among residents in community long-term-care facilities in Spain. Clin Microbiol Infect 14:867–872
Tacconelli E, Cataldo MA, De Pascale G, Manno D, Spanu T, Cambieri A et al (2008) Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. J Antimicrob Chemother 62:1130–1137
Depuydt PO, Vandijck DM, Bekaert MA, Decruyenaere JM, Blot SI, Vogelaers DP et al (2008) Determinants and impact of multidrug antibiotic resistance in pathogens causing ventilator-associated-pneumonia. Crit Care 12:R142
Von Korff M, Wagner EH, Saunders K (1992) A chronic disease score from automated pharmacy data. J Clin Epidemiol 45:197–203
McGregor JC, Kim PW, Perencevich EN, Bradham DD, Furuno JP, Kaye KS et al (2005) Utility of the chronic disease score and Charlson comorbidity index as comorbidity measures for use in epidemiologic studies of antibiotic-resistant organisms. Am J Epidemiol 161:483–493
Quach S, Hennessy DA, Faris P, Fong A, Quan H, Doig C (2009) A comparison between the APACHE II and Charlson index score for predicting hospital mortality in critically ill patients. BMC Health Serv Res 9:129
Combes A, Luyt CE, Fagon JY, Wolff M, Trouillet JL, Chastre J (2006) Impact of piperacillin resistance on the outcome of Pseudomonas ventilator-associated pneumonia. Intensive Care Med 32:1970–1978
Conflict of interest
The study was conducted with no funding. There is no potential conflict of interest of any nature related to the submitted manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article was partially presented at the 49th Infectious Diseases Medical Congress, Microbiology Medical Congress (ICAAC), San Francisco, CA, USA, 12th–15th September 2009.
Rights and permissions
About this article
Cite this article
Debby, B.D., Ganor, O., Yasmin, M. et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis 31, 1811–1817 (2012). https://doi.org/10.1007/s10096-011-1506-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1506-5