Abstract
Introduction
In 2020, the Italian Medicines Agency (AIFA) approved the reimbursement of calcitonin gene-related peptide (CGRP) pathway monoclonal antibodies (mAbs), including fremanezumab, in patients with a Migraine Disability Assessment Scale (MIDAS) score ≥ 11, with prescription renewals for up to 12 months in patients with ≥ 50% reduction in MIDAS score at Months 3 and 6. In this sub-analysis of the Pan-European Real Life (PEARL) study, we provide real-world data on fremanezumab use in Italian routine clinical practice (EUPAS35111).
Methods
This first interim analysis for Italy was conducted when 300 enrolled adult patients with episodic or chronic migraine (EM, CM) completed 6 months of treatment with fremanezumab. The primary endpoint is the proportion of patients achieving ≥ 50% reduction in monthly migraine days (MMD) across the 6 months post-fremanezumab initiation. Secondary endpoints include: proportion of patients achieving ≥ 50% reduction in MIDAS score at Months 3 and 6, and mean change from baseline across Months 1–6 in MMD and headache-related disability. Safety was assessed through adverse events (AEs) reported.
Results
Of 354 patients enrolled at Italian centers, 318 (EM, 35.5%, CM, 64.5%) were included in the effectiveness analysis. Of patients with available data, 109 (61.2%) achieved the primary endpoint. 61.0% and 65.1% achieved ≥ 50% reduction in MMDs at Months 3 and 6, respectively; 79.9% and 81.0% experienced ≥ 50% reduction in MIDAS at the same timepoints.
Conclusion
Fremanezumab was effective and well-tolerated over the first 6 months of treatment, with approximately 80% of patients meeting Italian criteria for treatment continuation at Months 3 and 6.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is one of the most prevalent causes of disability worldwide [1, 2]. Due to an impaired ability to perform daily activities, negative impacts on family life, loss of work productivity, reduced educational and career potential, and high healthcare resource utilization, migraine is associated with high disease burden and substantially reduced health-related quality of life (HRQoL) [3,4,5,6,7,8]. Furthermore, interictal burden and high levels of anticipatory anxiety are common in patients with episodic (< 15 headache days per month) and chronic (≥ 15 headache days per month for > 3 months, at least eight of which meet the International Classification of Headache Disorders [ICHD-3] criteria for migraine) migraine (EM and CM), and can lead to avoidance behaviors [9,10,11,12].
Reduction in monthly migraine days (MMD) is commonly used to assess the efficacy of migraine preventive drugs in controlled clinical trials [8]. However, because migraine touches so many aspects of a patient’s life, it is important to look beyond migraine frequency to understand the full scope of drug benefits, especially in everyday practice [6]. Limiting focus to the symptoms of migraine can also lead to shortcomings in a multifaceted approach to migraine treatment [6]. Two main scales are available to assess the impact of migraine on a patient’s life: the Migraine Disability Assessment Scale (MIDAS) and the 6-item Headache Impact Test (HIT-6) [13, 14]. MIDAS is a self-administered questionnaire, which includes five disability-related questions assessing a 3-month period. Responses to individual MIDAS questions and summary scores have shown high reliability in population-based studies of migraine and headache sufferers [13, 15].
Migraine prevention with calcitonin gene-related peptide (CGRP) pathway monoclonal antibodies (mAbs) reduces migraine days and improves HRQoL in patients with migraine. In phase 3 randomized controlled trials (RCTs) and relative long-term open-label extensions, monthly and quarterly doses of fremanezumab have demonstrated efficacy and safety in adults with both EM and CM, including those with documented inadequate response to 2–4 classes of prior preventive migraine medications in the past 10 years [16,17,18,19,20,21]. Furthermore, greater improvements in HRQoL and MIDAS score from baseline have been reported in patients receiving fremanezumab over 12 months [21, 22]. These patients also reported high levels of satisfaction, reduced anxiety, and increased quality of time spent with others [23]. In addition, data from real-world evidence (RWE) studies, which play an important role in supporting clinical decision making and driving treatment guidelines, have shown that fremanezumab has led to improvements in disability outcomes in adults with migraine in Europe [24,25,26,27,28,29].
In 2020, the Italian Medicines Agency (AIFA) established specific reimbursement conditions for CGRP pathway mAbs, including fremanezumab, for the preventive treatment of migraine in patients with high frequency EM (HFEM [8–14 migraine days per month]) and CM. To obtain reimbursement, a patient must have failed ≥ 3 other preventive treatments (e.g., β-blockers, tricyclic antidepressants, antiepileptics and onabotulinumtoxinA [the latter only for CM]) due to lack of efficacy or tolerability, and must have a MIDAS score before treatment of ≥ 11 points. Furthermore, AIFA requires a ≥ 50% reduction in MIDAS score from baseline at Months 3 and 6 to authorize renewal of the prescription with reimbursement for up to 12 months of treatment [30, 31]. In Italy, MIDAS is therefore considered both as a measure of drug effectiveness and of migraine-related disability, and represents a limiting factor in treatment continuation.
The Pan-European Real Life (PEARL; EUPAS35111) study is an ongoing, prospective, non-interventional, observational, phase 4 study, which aims to evaluate the effectiveness and safety of fremanezumab in a diverse European population with EM and CM, including individuals who have switched from another CGRP pathway mAb treatment. With a large cohort of patients and an observational period of 24 months, PEARL is the largest real-world data generation study for fremanezumab with a long duration of follow-up. This first interim analysis, which includes only Italian patient data, aims to provide RWE of fremanezumab usage in routine clinical practice according to Italian reimbursement criteria.
Methods
The complete protocol for the PEARL study, including all primary, secondary, and exploratory endpoints, has been published previously [32]. Here we report the methodology used for the first interim analysis in Italy.
Study oversight
The protocol has been approved by the Independent Ethics Committee/Institutional Review Board in all 11 participating European countries (Czech Republic, Denmark, Finland, Greece, Italy, Norway, Portugal, Spain, Sweden, Switzerland, and the UK), as required by local regulations, and all relevant local data protection laws are followed. Informed consent was obtained from all patients before inclusion in the study; patients agreed for their clinical data to be recorded anonymously, retaining the right to withdraw their consent at any time during the study [32].
Study design
PEARL is a 24-month, phase 4, multicenter, pan-European, prospective, observational study. As PEARL is a non-interventional, prospective study, no study procedures are performed above the patients’ real-world, routine clinical practice experience. The aim of the study is to evaluate the effectiveness, safety, and tolerability of fremanezumab treatment in adult patients with EM or CM in a real-world clinical setting across Europe. PEARL is currently being conducted in 87 sites across 11 European countries: 30 of these sites are located in Italy.
Participants
Eligible patients are adults (≥ 18 years) diagnosed with CM (≥ 15 headache days per month for > 3 months, ≥ 8 of which meet the International Classification of Headache Disorders criteria for migraine) or EM (< 15 headache days per month), who have been prescribed subcutaneous fremanezumab at doses of 225 mg monthly or 675 mg quarterly [32, 33]. Patients must have ≥ 21 days of paper or electronic headache diary data in the 28 days prior to fremanezumab treatment initiation, and must be willing to continue to record information on their headaches throughout the study period. The published PEARL protocol contains the full inclusion and exclusion criteria details [32].
The PEARL study has enrolled a total of 1140 patients, who will be followed for a 24-month observational period. The first patient was screened and enrolled in August 2020, while the last patient is expected to complete the study in early 2024. Enrollment in Italy started in February 2021. Interim analyses for the full patient population are scheduled for when 300, 500, and all enrolled patients have completed 6 months of treatment, and when all enrolled patients have completed 12 months of treatment [32, 34]. The final analysis is planned for late 2024 [32]. This first interim analysis in Italy was performed after 300 enrolled patients completed 6 months of treatment (data cut-off: 10 June 2022).
Study procedures
The study procedures described herein relate to all patients enrolled in the PEARL study. Patients are asked to maintain a written daily headache diary as part of their routine disease management during the 28-day baseline period and throughout the entire observational period of the study. Headache diaries can capture information on headache frequency, severity, duration, characteristics, and concomitant preventive and acute migraine medication use. Data are recorded and analyzed based on the features captured in individual diaries prior to enrollment. Patients are also invited to record the occurrence of any adverse event (AE). MIDAS and HIT-6 scores are captured at baseline and throughout the treatment period for all patients. Throughout the PEARL study period, physicians are recommended to schedule visits with patients at least every 3 months (± 15 days) for a total of nine visits, or as part of routine clinical practice and disease management, and at the discretion of the treating physician (Supplementary Fig. 1). Patients who are treated with a newly prescribed migraine preventive treatment following discontinuation of fremanezumab are excluded from the study. All other patients who discontinue fremanezumab treatment will be documented further during the observational period according to the visit schedule in local clinical practice and are encouraged by their treating physician to complete daily headache diaries as per guidelines and routine disease management. Treatment with fremanezumab may be resumed at any time, depending on the agreement between the patient and their treating physician. All headache diary data will be collected, regardless of a missed clinic visit. Patients could be excluded from the study for one or more reasons, including having unsigned baseline visits, < 4 migraine days in the baseline period being documented, or < 10 diary entries documented after the first dose of fremanezumab [32].
Assessment of outcomes
For all outcome measures, baseline is defined as the 28-day period prior to initiating fremanezumab treatment, as recorded through patient diary entries from this period. The primary endpoint is the proportion of patients who reach ≥ 50% reduction from baseline in average MMD across the 6-month period after fremanezumab initiation. Secondary clinical effectiveness endpoints, evaluated at Months 3 and 6 of the 24-month follow-up period, include the mean change from baseline in: disability scores, as measured by MIDAS and HIT-6; reduction in MMD; and the average monthly days of acute migraine medication use, including the proportion of patients achieving ≥ 50% reduction in mean number of days with triptan use.
The MIDAS questionnaire is designed to quantify migraine-related disability, with a scoring system assigned as: 0–5: little or no disability; 6–10: mild disability; 11–20: moderate disability; and > 20: severe disability [35]. The HIT-6 score is a questionnaire designed to help individuals with migraine describe and communicate how they feel and explain what they cannot do because of their headaches. While all individuals who have a HIT-6 score of ≥ 50 are recommended to see a doctor, the scoring system states: 50–55: some impact; 56–59: substantial impact; and ≥ 60: severe impact on a patient’s life [36]. Clinically meaningful reductions in MIDAS and HIT-6 scores are defined as at least 4.5-point and 8-point reductions from baseline, respectively [37, 38]. The safety of fremanezumab treatment is evaluated based on the documentation of AEs reported in clinical practice [32].
Statistical methods
The full analysis set (FAS) includes all patients enrolled from Italian centers who have ≥ 10 days of recorded data between treatment initiation and the last documented follow-up visit. Effectiveness data are analyzed in the FAS using patient-reported outcome measures (PROMs) from patient diaries and validated headache-related disability tools specified above [32].
All variables of the PEARL study are summarized descriptively. Continuous variables are analyzed with descriptive statistics for their actual values and changes from baseline at each visit, whilst for categorical variables, frequency, and percentage are provided. Full details of the statistical analysis can be found within the published PEARL study protocol [32]. Data for primary, secondary, and exploratory objectives are presented as both mean values across timepoints and as mean changes from baseline. The mean value is the average for all participants at a given time point, whereas the mean change from baseline measures the change between two time points for each individual participant. Therefore, the number of participants for the mean change is typically lower than change from baseline as data must be available for the individual participants at both time points.
Results
Study population
The cut-off date for the first interim analysis data collection in Italy was 10 June 2022. At the data cut-off, 354 (31.1%) of the 1140 patients in the PEARL study were enrolled at Italian centers. All 354 patients from Italy were included in the safety analysis set (SAS) for this analysis and 318 were included in the FAS: 205 of whom (64.5%) had CM and 113 (35.5%) had EM. Of the 318 patients in the FAS, eight terminated the study and two discontinued fremanezumab but continued to be followed in the study (Fig. 1).
Patient disposition. aPatient can be excluded for one or more of the following reasons: Baseline visit is unsigned, less than 4 migraine days in the baseline period are documented, less than 10 diary entries are documented after the first dose of fremanezumab. CM = chronic migraine, EM = episodic migraine, FAS = full analysis set, SAS = safety analysis set
Patients in the FAS were mainly females (n = 263 [82.7%]), and most patients were aged 35 to < 65 years of age (n = 243 [76.4%]). A total of 298 (93.7%) patients received only monthly fremanezumab dosing, 14 (4.4%) patients received only quarterly fremanezumab dosing, and six (1.9%) patients received both dosing schedules (on separate occasions) throughout the treatment period. MIDAS scores ≥ 11 points (indicating at least moderate disability) at baseline were reported in 98.4% of patients. Anticonvulsants and tricyclic antidepressants were the most common migraine preventive treatment previously used by patients with EM (90 [79.6%] and 98 [86.7%] patients, respectively) and CM (175 [85.4%] and 190 [92.7%] patients, respectively). Psychiatric disorders (22.0%) and metabolism and nutrition disorders (17.0%) were the most frequently reported pre-existing conditions, followed by vascular disorders (14.5%) and endocrine disorders (13.2%, Table 1).
Primary endpoint: ≥ 50% decrease in MMD over 6 months
Of 178 patients with available data, 109 (61.2%) achieved ≥ 50% reduction in MMD across the first 6 months of fremanezumab treatment, (63.9% for EM and 59.8% for CM, Fig. 2).
Secondary efficacy endpoints
≥ 50% reduction in MMD at Month 3 and Month 6
Of patients with data available for reduction in MMD at each time-point, 61.0% at Month 3 and 65.1% at Month 6 achieved a reduction of ≥ 50% in mean MMD after fremanezumab initiation; this was numerically higher in those with EM than CM at Month 3 (66.7% vs 57.6%) and lower in EM than CM at Month 6 (59.0% vs 68.4%, Supplementary Fig. 2).
Change from baseline in MMD at Month 3 and Month 6
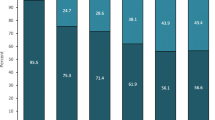
For total patients, a 54.4% and 62.0% reduction in the average number of MMD from baseline was reported at Months 3 and 6, respectively. These reductions were 57.9% and 59.8% for EM, and 53.2% and 62.9% for CM at Months 3 and 6, respectively (Fig. 3).
MMD at baseline, Month 3 and Month 6 (a) and change from baseline in MMD at Month 3 and Month 6 (b) by migraine type. Mean change from baseline measures the change between two time points for each individual patient, whereas the mean is the average for all patients at a given time point. CM = chronic migraine, EM = episodic migraine, MMD = monthly migraine days
Change from baseline in disability scores
A ≥ 50% decrease in MIDAS score from baseline was achieved by 238 (79.9%) and 179 (81.0%) patients at Months 3 and 6, respectively. The proportion of patients achieving ≥ 50% reduction in MIDAS score was numerically higher in patients with EM versus CM at Month 3 (82.4% vs 78.4%) and the same at Month 6 (81.0% for both, Supplementary Fig. 3).
At Month 3, we observed 65.9%, 73.2%, and 63.2% reductions in MIDAS score from baseline for total, EM and CM patients, respectively. At Month 6, these reductions from baseline were 74.3%, 76.9%, and 73.4% for total, EM and CM patients, respectively (Fig. 4). The mean reduction in HIT-6 score from baseline observed at Months 3 and 6 were –9.7 and –11.8 for the total population, –10.9 and –12.3 for EM, and –9.1 and –11.6 for CM.
MIDAS score at baseline, Month 3 and Month 6 (a) and change from baseline in MIDAS score at Month 3 and Month 6 (b) by migraine type. Mean change from baseline measures the change between two time points for each individual patient, whereas the mean is the average for all patients at a given time point. CM = chronic migraine, EM = episodic migraine, MIDAS = Migraine Disability Assessment Scale
Change from baseline in average monthly days of acute migraine medication use
The monthly average number of days with any acute migraine medication in the total population decreased by 61.7% and 68.4% from baseline at Months 3 and 6, respectively. For patients with EM, reductions from baseline of 64.6% were observed at both Months 3 and 6, and for CM, reduction from baseline in days with acute medication use was 60.5% at Month 3 and 70.4% at Month 6 (Supplementary Fig. 4). The proportion of patients achieving ≥ 50% reduction in mean number of days with triptan use was 74.5% at Month 3 and 70.4% at Month 6.
Safety
Of 354 patients in the SAS, 45 (12.7%) reported ≥ 1 AE. In total, 110 AEs were reported within the first 6 months of treatment: 47 AEs were reported in 18 patients with EM (14.4% of patients), while 63 AEs were reported in 27 patients with CM (11.8% of patients). AEs leading to discontinuation of fremanezumab were reported in 17 patients (4.8%) in the SAS, with eight patients prematurely terminating the study and nine patients continuing study observation (Table 2).
The most frequently reported AEs were assigned to the system organ class ‘general disorders and administration site conditions’ (6.2% of patients in the SAS): most commonly these were injection site erythema (1.7%) and injection site pruritus (1.4%). Gastrointestinal disorders were reported by 4.0% of patients in the SAS, including constipation (2.3%) and nausea (1.4%) (Supplementary Table 1).
Discussion
In this RWE study, 354 patients with migraine enrolled from centers in Italy were treated with fremanezumab. Of these, 64.7% had CM and 98.4% met Italian reimbursement criteria at enrollment (≥ 8 MMD, MIDAS score ≥ 11, and ≥ 3 previous preventive medication failures) [30]. These data indicate that fremanezumab was effective and well-tolerated over the first 6 months of treatment, with 79.9% and 81.0% of patients meeting the Italian criteria for treatment continuation (≥ 50% reduction in MIDAS score) at 3 and 6 months, respectively. The efficacy of fremanezumab was also proven through clinically relevant reductions in MMD, HIT-6 scores and a reduction in the use of acute migraine medicine over 6 months. No new safety signals were observed throughout the study duration, with gastrointestinal AEs, including constipation, lower compared with other RWE studies on CGRP pathway mAbs [39, 40].
MIDAS is a unique efficacy parameter for Italy, as reductions in MMD are often used as a benchmark for CGRP pathway mAb continuation both throughout Europe and globally [41]. In this analysis, the percentage of patients achieving ≥ 50% reduction in MIDAS score was substantially higher than MMD response, where 61.0% and 56.1% of patients achieved ≥ 50% reduction in MMD at Months 3 and 6, respectively. Similarly, a long-term effectiveness study of three CGRP pathway mAbs found that ≥ 50% reduction in MIDAS score was achieved by 89.5% of patients compared with 36.4%–56.8% for MMD at Month 6, with authors concluding that the MIDAS score was the most advantageous efficacy scale in this setting [42]. In this context, however, a RWE study of 77 patients with CM treated with erenumab showed that a ≥ 50% reduction in MIDAS score at 3 months excluded more than one third of responders at Month 12, thus suggesting that the combined use of a reduction in MIDAS score and MMD could better reflect the proportion of patients who can benefit from treatment with CGRP pathway mAbs [43]. This discrepancy may be explained by the fact that MIDAS score indirectly reflects the intensity of migraine, while MMD reflect purely the number of days with migraine, regardless of their intensity [13].
RWE studies in Italy, the United States, and the United Kingdom have also demonstrated real-world effectiveness of fremanezumab over 4–6 months, including reductions in HIT-6 and MIDAS scores in patients with previous migraine preventive failures. These results are consistent with this PEARL analysis, indicating that fremanezumab effectiveness remains high in RWE studies in patients for whom multiple migraine preventive treatments have failed. No new safety findings compared with RCTs have been identified in these studies [26, 44,45,46].
One main strength of this study includes the real-world setting. Real-world studies are typically more inclusive than RCTs and involve a broader patient population. Regulatory bodies have recognized real-world studies as highly useful and complimentary to RCTs, by offering insight across a more diverse group of patients [47]. In addition, the primary endpoint of ≥ 50% reduction in MMD over 6 months, instead of the evaluation at Month 6, provides more comprehensive and detailed information about the persistence of fremanezumab effectiveness, due to the ability to estimate response over this time-period using data available from the previous months [32]. Furthermore, studies such as PEARL allow the exploration of disease management in different countries with varying reimbursement criteria: the involvement of 30 centers from Italy provides a broad overview of fremanezumab effectiveness and safety across a large patient cohort.
The results may have been limited by the fact this study was initiated during the COVID-19 pandemic. During this period, all hospitals in Italy had limited access; therefore, a number of treatment interruptions may have occurred, resulting in some gaps in data collection. Additionally, data collection through patient headache diaries relies on the accuracy of the recorder and risks human error or the reporting of perceived ‘favorable’ outcomes. As is common in real-world studies, the percentage of missing data is higher than would be expected with a RCT [48]. As a result, data at 6 months and beyond should be interpreted with caution due to reduced sample size. More data will be collected and presented in future analyses. It should also be noted that as only 4.4% of patients included in this analysis used quarterly fremanezumab dosing, these data do not reflect any differences between monthly and quarterly dosing schedules.
Observational RWE studies such as PEARL are vital to complement information about CGRP pathway mAbs in migraine prevention. The data from this first interim analysis of an Italian cohort showed fremanezumab to be efficacious in patients who have more treatment failures than those commonly seen in RCTs. Overall, the high percentage of patients achieving ≥ 50% reduction in MIDAS score compared with MMD suggests that the reduction in MMD alone may not be suitable to capture the extent of the benefit associated with the use of fremanezumab in the real-world setting, as it does not provide information on migraine-related disability. Future PEARL analyses will continue to look at the long-term efficacy and safety of fremanezumab treatment, both in the full study population, in the subgroup of patients enrolled from Italian centers, and in different patient populations, including those with older age and comorbid conditions.
Data Availability
The data sets used and/or analyzed for the study described in this manuscript are available upon reasonable request. Qualified researchers may request access to patient level data and related study documents including the study protocol and the statistical analysis plan. Patient level data will be de-identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please visit www.clinicalstudydatarequest.com to make your request.
References
Steiner TJ, Stovner LJ, Jensen R et al (2020) Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain 21:137. https://doi.org/10.1186/s10194-020-01208-0
GBD 2016 Neurology Collaborators (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Safiri S, Pourfathi H, Eagen A et al (2022) Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain 163:e293–e309. https://doi.org/10.1097/j.pain.0000000000002275
Ashina M, Katsarava Z, Phu Do T et al (2021) Migraine: epidemiology and systems of care. Lancet 397:1485–1495. https://doi.org/10.1016/S0140-6736(20)32160-7
Vo P, Fang J, Bilitou A et al (2018) Patients’ perspective on the burden of migraine in Europe: a cross-sectional analysis of survey data in France, Germany, Italy, Spain and the United Kingdom. J Headache Pain 19(1):82. https://doi.org/10.1186/s10194-018-0907-6
Steiner TJ, Stovner LJ, Katsarava Z et al (2014) The impact of headache in Europe: principal results of the Eurolight project. J Headache Pain 15(1):31. https://doi.org/10.1186/1129-2377-15-31
Stovner LJ, Andrée C, Committee ES (2008) Impact of headache in Europe: a review for the Eurolight project. J Headache Pain 9(3):139–146. https://doi.org/10.1007/s10194-008-0038-6
The International Headache Society (IHS) (2021) International Classification of Headache Disorders (ICHD-3). The International Headache Society. https://ichd-3.org/. Accessed 14 June 2023
Lampl C, Thomas H, Stovner LJ et al (2016) Interictal burden attributable to episodic headache: findings from the Eurolight project. J Headache Pain 17:9. https://doi.org/10.1186/s10194-016-0599-8
Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition (2004) Cephalalgia 24(Suppl. 1):1–160. https://doi.org/10.1111/j.1468-2982.2003.00824.x
Hubig L, Smith T, Williams E et al (2022) Measuring interictal burden among people affected by migraine: a descriptive survey study. J Headache Pain 23:97. https://doi.org/10.1186/s10194-022-01467-z
Estave PM, Beeghly S, Anderson R et al (2021) Learning the full impact of migraine through patient voices: A qualitative study. Headache 61:1004–1020. https://doi.org/10.1111/head.14151
Stewart WF, Lipton RB, Kolodner KB et al (2000) Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 88(1):41–52. https://doi.org/10.1016/S0304-3959(00)00305-5
Sauro KM, Rose MS, Becker WJ et al (2010) HIT-6 and MIDAS as measures of headache disability in a headache referral population. Headache 50(3):383–395. https://doi.org/10.1111/j.1526-4610.2009.01544.x
Stewart WF, Lipton RB, Kolodner K et al (1999) Reliability of the Migraine Disability Assessment (MIDAS) score in a population based sample of headache sufferers. Cephalalgia 19:107–114. https://doi.org/10.1046/j.1468-2982.1999.019002107.x
Ferrari MD, Diener HC, Ning X et al (2019) Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): A randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 394:1030–1040. https://doi.org/10.1016/S0140-6736(19)31946-4
Dodick DW, Silberstein SD, Bigal ME et al (2018) Effect of fremanezumab compared with placebo for prevention of episodic migraine: A randomized clinical trial. JAMA 319:1999–2008. https://doi.org/10.1001/jama.2018.4853
Silberstein SD, Cohen JM, Seminerio MJ et al (2020) The impact of fremanezumab on medication overuse in patients with chronic migraine: subgroup analysis of the HALO CM study. J Headache Pain 21(1):114. https://doi.org/10.1186/s10194-020-01173-8
Silberstein SD, Dodick DW, Bigal ME et al (2017) Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med 377:2113–2122. https://doi.org/10.1056/NEJMoa1709038
Lipton RB, Cohen JM, Bibeau K et al (2020) Reversion from chronic migraine to episodic migraine in patients treated with fremanezumab: post hoc analysis from HALO CM study. Headache 60:2444–2453. https://doi.org/10.1111/head.13997
Goadsby PJ, Silberstein SD, Yeung PP et al (2020) Long-term safety, tolerability, and efficacy of fremanezumab in migraine: A randomized study. Neurology 95:e2487–e2499. https://doi.org/10.1212/WNL.0000000000010600
Blumenfeld AM, Stevanovic DM, Ortega M et al (2020) No “wearing-off effect” seen in quarterly or monthly dosing of fremanezumab: subanalysis of a randomized long-term study. Headache 60:2431–2443. https://doi.org/10.1111/head.13994
Buse DC, Gandhi SK, Cohen JM et al (2020) Improvements across a range of patient-reported domains with fremanezumab treatment: results from a patient survey study. J Headache Pain 21:109. https://doi.org/10.1186/s10194-020-01177-4
Barbanti P, Egeo G, Aurilia C et al (2023) Early and sustained efficacy of fremanezumab over 24-weeks in migraine patients with multiple preventive treatment failures: the multicenter, prospective, real-life FRIEND2 study. J Headache Pain 24:30. https://doi.org/10.1186/s10194-023-01561-w
Food and Drug Administration (2022) Real-world evidence. FDA. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence. Accessed 13 Oct 2022
Barbanti P, Egeo G, Aurilia C et al (2022) Fremanezumab in the prevention of high-frequency episodic and chronic migraine: a 12-week, multicenter, real-life, cohort study (the FRIEND study). J Headache Pain 23:46. https://doi.org/10.1186/s10194-022-01396-x
Cheng F, Wu Q, Hussain M et al (2022) Efficacy of fremanezumab in resistant and refractory chronic migraine patients: real-world data from the Hull Migraine Clinic, UK. Adv Neur Neur Sci 5(2):45–67. https://doi.org/10.21203/rs.3.rs-1340639/v1
Nasergivehchi S, Cheng F, Hussain M et al (2022) One year outcome of fremanezumab in refractory chronic migraine patients: real-world data from the Hull. Cephalalgia 42(1_suppl):130. https://doi.org/10.1177/03331024221123848
Straube A, Broessner G, Gaul C et al (2022) Response to fremanezumab for preventive treatment in migraine in routine clinical practice: first data from the FINESSE study. Headache 62:160–161. https://doi.org/10.1111/head.14317
Italian Medicines Agency (AIFA) (2020) Attivazione web e pubblicazione schede di monitoraggio. AIFA. https://www.aifa.gov.it/en/-/attivazione-web-e-pubblicazione-schede-di-monitoraggio-registro-aimovig. Accessed 6 June 2023
Silberstein SD, Lipton RB, Sliwinski M (1996) Classification of daily and near-daily headaches: Field trial of revised IHS criteria. Neurology 47:871–875. https://doi.org/10.1212/wnl.47.4.871
Ashina M, Amin FM, Kokturk P et al (2021) PEARL study protocol: a real-world study of fremanezumab effectiveness in patients with chronic or episodic migraine. Pain Manag 11:647–654. https://doi.org/10.2217/pmt-2021-0015
IHC (2018) The international classification of headache disorders, 3rd edition. Cephalalgia 38(1):1–211. https://doi.org/10.1177/0333102417738202
Ashina M, Mitsikostas D, Amin F et al (2022) Effectiveness of fremanezumab for preventive treatment of migraine: the observational PEARL study. 29:192–193. https://doi.org/10.1111/ene.15465
Stewart WF, Lipton RB, Dowson AJ et al (2001) Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 56(6 Suppl 1):S20–S28. https://doi.org/10.1212/wnl.56.suppl_1.s20
QualityMetric, Inc. and GlaxoSmithKline Group of Companies. HIT-6 Scoring Interpretation English Version 1.1. https://bash.org.uk/wp-content/uploads/2012/07/English.pdf. Accessed 1 December 2022
Caralho GF, Luedtke K, Braun T (2021) Minimal important change and responsiveness of the Migraine Disability Assessment Score (MIDAS) questionnaire. J Headache Pain 22:126. https://doi.org/10.1186/s10194-021-01339-y
Castien RF, Blankenstein AH, van der Windt ADWM et al (2012) Minimal clinically important change on the Headache Impact Test-6 questionnaire in patients with chronic tension-type headache. Cephalalgia 32:710–714. https://doi.org/10.1177/0333102412449933
Cullum CK, Phu Do T, Ashina M et al (2022) Real-world long-term efcacy and safety of erenumab in adults with chronic migraine: a 52-week, single-center, prospective, observational study. J Headache Pain 23:61. https://doi.org/10.1186/s10194-022-01433-9
Sacco S, Amin FM, Ashina M et al (2022) European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention – 2022 update. J Headache Pain 23:67. https://doi.org/10.1186/s10194-022-01431-x
National Institute for Health and Care Excellence (NICE) (2022) Fremanezumab for preventing migraine. https://www.nice.org.uk/guidance/ta764/chapter/1-Recommendations. NICE. Accessed 6 June 2023
Iannone LF, Fattori D, Benemei S et al (2022) Long-term effectiveness of three anti-CGRP monoclonal antibodies in resistant chronic migraine patients based on the MIDAS score. CNS Drugs 36:191–202. https://doi.org/10.1007/s40263-021-00893-y
De Icco R, Vaghi G, Allena M et al (2022) Does MIDAS reduction at 3 months predict the outcome of erenumab treatment? A real-world, open-label trial. J Headache Pain 23:123. https://doi.org/10.1186/s10194-022-01480-2
Driessen MT, Cohen JM, Patterson-Lomba O et al (2022) Real-world effectiveness of fremanezumab in migraine patients initiating treatment in the United States: results from a retrospective chart study. J Headache Pain 23:47. https://doi.org/10.1186/s10194-022-01411-1
Cheng F, Wu Q, Hussain M et al (2022) Efficacy of fremanezumab in resistant and refractory chronic migraine patients: real-world data from the Hull Migraine Clinic, UK. Adv Neurol Neurosci 5:45–67
Ferrari MD, Diener HC, Ning X et al (2019) Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 394(10203):1030–1040. https://doi.org/10.1016/S0140-6736(19)31946-4
Nazha B, Yang JC, Owonikoko TK (2021) Benefits and limitations of real-world evidence: lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncol 17:965–977. https://doi.org/10.2217/fon-2020-0951
Liu M, Qi Y, Wang W et al (2022) Toward a better understanding about real-world evidence. Eur J Hosp Pharm 29:8–11. https://doi.org/10.1136/ejhpharm-2021-003081
Acknowledgements
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Jessica Amps of Ashfield MedComms, an Inizio company, and funded by Teva Pharmaceuticals. We thank the PEARL study group for their contribution to the project: Anna Ambrosini, Monica Bandettini, Marco Bartolini, Chiara Benedetto, Filippo Brighina, Sabina Cevoli, Gianluca Coppola, Roberto De Simone, Paola Di Fiore, Florindo D’Onofrio, Sara Gori, Antonio Granato, Simona Guerzoni, Rosario Iannacchero, Stefano Messina, Francesco Perini, Maria Pia Prudenzano, Innocenzo Rainero, Renata Rao, Ester Reggio, Paola Sarchielli, Giuliano Sette, Susanna Usai, Mariarosaria Valente, and Fabrizio Vernieri.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical approval and Informed consent
The protocol has been approved by the Independent Ethics Committee/Institutional Review Board in all 11 participating European countries (Czech Republic, Denmark, Finland, Greece, Italy, Norway, Portugal, Spain, Sweden, Switzerland, and the UK), as required by local regulations, and all relevant local data protection laws are followed. Informed consent was obtained from all patients before inclusion in the study; patients agreed for their clinical data to be recorded anonymously, retaining the right to withdraw their consent at any time during the study.
Conflict of interest
CT has received consulting fees or speaking honoraria from AbbVie, Dompé, Lilly, Lundbeck, Novartis, and Pfizer; has received financial support for attending meetings from Lundbeck and Ipsen; is the past-President of the International Headache Society.
PB has received consulting fees from AbbVie, Allergan, Angelini, Assosalute, Bayer, Eli-Lilly, Fondazione Ricerca e Salute, Lundbeck, MSD, Novartis, Teva, Visufarma, and Zambon; has received payment or honoraria from AbbVie, Allergan, Angelini, Bayer, Eli-Lilly, Lundbeck, MED, MSD, New Penta, Novartis, Stx-Med, Teva, Visufarma, and Zambon; has received support for attending meetings and/or travel from Lundbeck, Novartis, Teva, and Visufarma; has participated on a data safety monitoring board or advisory board for AbbVie, Allergan, Angelini, Bayer, Eli-Lilly, Lundbeck, New Penta, Novartis, Stx-Med, Teva, Visufarma, and Zambon; and has received other financial or non-financial interests from AbbVie, Amgen, Alder, Allergan, Biohaven, ElectroCore, Eli-Lilly, GSK, Lundbeck, New Penta, Noema Pharma, Novartis, and Teva.
MC is an employee of Teva Pharmaceuticals and has received stock or stock options from Teva.
SS has received grants or contracts from Novartis, and Uriach; has received consulting fees from AbbVie, Abbott, Allergan, AstraZeneca, Lilly, Lundbeck, NovoNordisk, and Pfizer; has received support for attending meetings and/or travel from Lilly, Lundbeck, Novartis, and Teva; is the President Elect for the European Stroke Organisation and second Vice-President for the European Headache Federation; and has received receipt of equipment, materials, drugs, medical writing gifts or other services from AbbVie, Allergan, and NovoNordisk.
AR has received speaker honoraria from Allergan, Lilly, Novartis, Teva, and Pfizer, and serves as an Associate Editor of Frontiers in Neurology (Headache Medicine and Facial Pain session).
CF has received payments or honoraria from AbbVie, Lilly, Lundbeck, Novartis, and Teva, and is the President of Anircef.
PK is an employee of Teva Pharmaceuticals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tassorelli, C., Barbanti, P., Finocchi, C. et al. The first interim analysis of Italian patients enrolled in the real-world, Pan-European, prospective, observational, phase 4 PEARL study of fremanezumab effectiveness. Neurol Sci 45, 2353–2363 (2024). https://doi.org/10.1007/s10072-024-07357-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-024-07357-3