Abstract
Objective
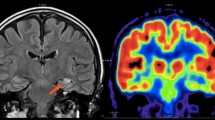
The aim of this investigation was to determine whether a correlation could be discerned between perfusion acquired through ASL MRI and metabolic data acquired via 18F-fluorodeoxyglucose (18F-FDG) PET in mesial temporal lobe epilepsy (mTLE).
Methods
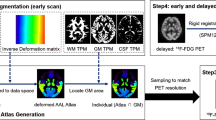
ASL MRI and 18F-FDG PET data were gathered from 22 mTLE patients. Relative cerebral blood flow (rCBF) asymmetry index (AIs) were measured using ASL MRI, and standardized uptake value ratio (SUVr) maps were obtained from 18F-FDG PET, focusing on bilateral vascular territories and key bitemporal lobe structures (amygdala, hippocampus, and parahippocampus). Intra-group comparisons were carried out to detect hypoperfusion and hypometabolism between the left and right brain hemispheres for both rCBF and SUVr in right and left mTLE. Correlations between the two AIs computed for each modality were examined.
Results
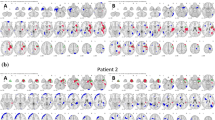
Significant correlations were observed between rCBF and SUVr AIs in the middle temporal gyrus, superior temporal gyrus, and hippocampus. Significant correlations were also found in vascular territories of the distal posterior, intermediate anterior, intermediate middle, proximal anterior, and proximal middle cerebral arteries. Intra-group comparisons unveiled significant differences in rCBF and SUVr between the left and right brain hemispheres for right mTLE, while hypoperfusion and hypometabolism were infrequently observed in any intracranial region for left mTLE.
Conclusion
The study’s findings suggest promising concordance between hypometabolism estimated by 18F-FDG PET and hypoperfusion determined by ASL perfusion MRI. This raises the possibility that, with prospective technical enhancements, ASL perfusion MRI could be considered an alternative modality to 18F-FDG PET in the future.



Similar content being viewed by others
Data Availability
The data will be available by sending request to the corresponding author, for integration into large cohort studies and meta data analyses.
References
Perucca P, Scheffer IE, Kiley M (2018) The management of epilepsy in children and adults. Med J Aust 208(5):226–233
Sisodiya SM et al (2022) The ENIGMA-Epilepsy working group: mapping disease from large data sets. Hum Brain Mapp 43(1):113–128
Nazem-Zadeh M-R et al (2016) MEG coherence and DTI connectivity in mTLE. Brain Topogr 29(4):598–622
Mahmoudi F et al (2018) Data mining MR image features of select structures for lateralization of mesial temporal lobe epilepsy. PLoS ONE 13(8):e0199137
Fallahi A et al (2022) Comparison of multimodal findings on epileptogenic side in temporal lobe epilepsy using self-organizing maps. Magn Reson Mater Phys, Biol Med 35(2):249–266
Mobarakeh NM et al (2019) The use of proton MR spectroscopy in epilepsy: a methodological review. Front Biomed Technol 6(1):1–21
Tomás J et al (2019) The predictive value of hypometabolism in focal epilepsy: a prospective study in surgical candidates. Eur J Nucl Med Mol Imaging 46(9):1806–1816
Steinbrenner M et al (2022) Utility of 18F-fluorodeoxyglucose positron emission tomography in presurgical evaluation of patients with epilepsy: A multicenter study. Epilepsia 63(5):1238–1252
Sone D et al (2019) Similar and differing distributions between 18F-FDG-PET and arterial spin labeling imaging in temporal lobe epilepsy. Front Neurol 10:318
Rahimzadeh H et al (2023) Alteration of intracranial blood perfusion in temporal lobe epilepsy, an arterial spin labeling study. Heliyon 9(4):e14854
Fan AP et al (2019) Identifying hypoperfusion in moyamoya disease with arterial spin labeling and an [15O]-water positron emission tomography/magnetic resonance imaging normative database. Stroke 50(2):373–380
Zaharchuk G, Fan A, Gulaka P, Guo J, Poston, K., Greicius M, ..., Zeineh M (2017l) Early uptake Amyloid PET imaging correlates strongly with cerebral blood flow based on arterial spin labeling MRI: a simultaneous PET/MRI study. In: Journal of Cerebral Blood Flow and Metabolism, vol 37. 2455 TELLER RD, THOUSAND OAKS, CA 91320 USA: SAGE PUBLICATIONS INC, pp 224-225.
Guo J (2023) Robust dual-module velocity-selective arterial spin labeling (dm-VSASL) with velocity-selective saturation and inversion. Magn Reson Med 89(3):1026–1040
Guo J, Das S, Hernandez-Garcia L (2021) Comparison of velocity-selective arterial spin labeling schemes. Magn Reson Med 85(4):2027–2039
Rahimzadeh H et al (2019) An efficient framework for accurate arterial input selection in DSC-MRI of glioma brain tumors. J Biomed Phys Eng 9(1):69
Lee DA et al (2021) Temporal lobe epilepsy with or without hippocampal sclerosis: structural and functional connectivity using advanced MRI techniques. J Neuroimaging 31(5):973–980
Zadeh HR, et al. (2017) Arterial input function selection in DSC-MRI of brain tumors using differential evaluation clustering method. In Proc. Intl. Soc. Mag. Reson. Med
Musiek ES et al (2012) Direct comparison of FDG-PET and ASL-MRI in Alzheimer’s disease. Alzheimer’s Dementia 8(1):51
Wolf RL et al (2001) Detection of mesial temporal lobe hypoperfusion in patients with temporal lobe epilepsy by use of arterial spin labeled perfusion MR imaging. Am J Neuroradiol 22(7):1334–1341
Lim Y-M et al (2008) Usefulness of pulsed arterial spin labeling MR imaging in mesial temporal lobe epilepsy. Epilepsy Res 82(2–3):183–189
Kojan M et al (2021) Arterial spin labeling is a useful MRI method for presurgical evaluation in MRI-negative focal epilepsy. Brain Topogr 34(4):504–510
Wang Y-H, et al. (2018) Comparison between simultaneously acquired arterial spin labeling and 18F-FDG PET in mesial temporal lobe epilepsy assisted by a PET/MR system and SEEG. NeuroImage: Clin 19: 824–830
Horváth R et al (2008) Brain lateralization and seizure semiology: ictal clinical lateralizing signs. Ideggyogyaszati Szemle 61(7–8):231–237
Marks WJ Jr, Laxer KD (1998) Semiology of temporal lobe seizures: value in lateralizing the seizure focus. Epilepsia 39(7):721–7264
Blair RD (2012) Temporal lobe epilepsy semiology. Epilepsy Res Treat 2012:751510. https://doi.org/10.1155/2012/751510
Mani J (2014) Video electroencephalogram telemetry in temporal lobe epilepsy. Ann Indian Acad Neurol 17(Suppl 1):S45
Chappell MA et al (2008) Variational Bayesian inference for a nonlinear forward model. IEEE Trans Signal Process 57(1):223–236
Buxton RB et al (1998) A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 40(3):383–396
Alsop DC et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73(1):102–116
Dolui S, et al. (2020) Arterial spin labeling versus 18F-FDG-PET to identify mild cognitive impairment. NeuroImage: Clin 25:102146
Galazzo IB, et al. (2016) Cerebral metabolism and perfusion in MR-negative individuals with refractory focal epilepsy assessed by simultaneous acquisition of 18F-FDG PET and arterial spin labeling. NeuroImage: Clin 11: 648–657
Verclytte S et al (2016) Cerebral hypoperfusion and hypometabolism detected by arterial spin labeling MRI and FDG-PET in early-onset Alzheimer’s disease. J Neuroimaging 26(2):207–212
Maldjian JA et al (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19(3):1233–1239
Nazem-Zadeh M-R, Elisevich K, Soltanian-Zadeh H (2018) Development of multimodal neuroimaging models for lateralization of temporal lobe epilepsy In: 2018 3rd Biennial South African Biomedical Engineering Conference (SAIBMEC), Stellenbosch, South Africa, pp 1–4. https://doi.org/10.1109/SAIBMEC.2018.8408603
Nazem-Zadeh MR, Elisevich KV, Schwalb JM, Bagher-Ebadian H, Mahmoudi F, Soltanian-Zadeh H (2014) Lateralization of temporal lobe epilepsy by multimodal multinomial hippocampal response-driven models. Journal of the neurological sciences 347(1–2):107–118. https://doi.org/10.1016/j.jns.2014.09.029
Nelissen N et al (2006) Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage 32(2):684–695
Guo X et al (2015) Asymmetry of cerebral blood flow measured with three-dimensional pseudocontinuous arterial spin-labeling MR imaging in temporal lobe epilepsy with and without mesial temporal sclerosis. J Magn Reson Imaging 42(5):1386–1397
Rahimzadeh H, Kamkar H, Hoseini-Tabatabaei N, Mobarakeh NM, Habibabadi JM, Hashemi-Fesharaki SS, Nazem-Zadeh MR (2023) Alteration of intracranial blood perfusion in temporal lobe epilepsy, an arterial spin labeling study. Heliyon 9(4):e14854. https://doi.org/10.1016/j.heliyon.2023.e14854
Jber M et al (2021) Temporal and extratemporal atrophic manifestation of temporal lobe epilepsy using voxel-based morphometry and corticometry: clinical application in lateralization of epileptogenic zone. Neurol Sci 42(8):3305–3325
Ahmadi ME et al (2009) Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. Am J Neuroradiol 30(9):1740–1747
Zhou X et al (2019) Disruption and lateralization of cerebellar–cerebral functional networks in right temporal lobe epilepsy: a resting-state fMRI study. Epilepsy Behav 96:80–86
Paldino MJ et al (2017) Comparison of the diagnostic accuracy of PET/MRI to PET/CT-acquired FDG brain exams for seizure focus detection: a prospective study. Pediatr Radiol 47(11):1500–1507
Acknowledgements
The authors would like to express their gratitude to the Iranian National Brain Mapping Laboratory (NBML), Tehran, Iran, for their invaluable assistance in data acquisition.
Funding
This work was financially supported by the Medical School of the Tehran University of Medical Sciences under Grant Number 98–3-101–45419, between the years 2019 and 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The studies involving human participants were conducted in accordance with the ethical standards set by the institutional and/or national research committee. The research also adhered to the principles outlined in the 1964 Helsinki Declaration and its subsequent amendments, or comparable ethical standards.
Consent to participate
All individual participants included in the study provided informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hossein Rahimzadeh and Hadi Kamkar equally contributed as co-first authors.
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahimzadeh, H., Kamkar, H., Ghafarian, P. et al. Exploring ASL perfusion MRI as a substitutive modality for 18F-FDG PET in determining the laterality of mesial temporal lobe epilepsy. Neurol Sci 45, 2223–2243 (2024). https://doi.org/10.1007/s10072-023-07188-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07188-8