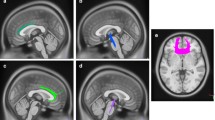
Abstract
Magnetoencephalography (MEG) is a noninvasive imaging method for localization of focal epileptiform activity in patients with epilepsy. Diffusion tensor imaging (DTI) is a noninvasive imaging method for measuring the diffusion properties of the underlying white matter tracts through which epileptiform activity is propagated. This study investigates the relationship between the cerebral functional abnormalities quantified by MEG coherence and structural abnormalities quantified by DTI in mesial temporal lobe epilepsy (mTLE). Resting state MEG data was analyzed using MEG coherence source imaging (MEG-CSI) method to determine the coherence in 54 anatomical sites in 17 adult mTLE patients with surgical resection and Engel class I outcome, and 17 age- and gender- matched controls. DTI tractography identified the fiber tracts passing through these same anatomical sites of the same subjects. Then, DTI nodal degree and laterality index were calculated and compared with the corresponding MEG coherence and laterality index. MEG coherence laterality, after Bonferroni adjustment, showed significant differences for right versus left mTLE in insular cortex and both lateral orbitofrontal and superior temporal gyri (p < 0.017). Likewise, DTI nodal degree laterality, after Bonferroni adjustment, showed significant differences for right versus left mTLE in gyrus rectus, insular cortex, precuneus and superior temporal gyrus (p < 0.017). In insular cortex, MEG coherence laterality correlated with DTI nodal degree laterality (\(R^{2} = 0.46; p = 0.003)\) in the cases of mTLE. None of these anatomical sites showed statistically significant differences in coherence laterality between right and left sides of the controls. Coherence laterality was in agreement with the declared side of epileptogenicity in insular cortex (in 82 % of patients) and both lateral orbitofrontal (88 %) and superior temporal gyri (88 %). Nodal degree laterality was also in agreement with the declared side of epileptogenicity in gyrus rectus (in 88 % of patients), insular cortex (71 %), precuneus (82 %) and superior temporal gyrus (94 %). Combining all significant laterality indices improved the lateralization accuracy to 94 % and 100 % for the coherence and nodal degree laterality indices, respectively. The associated variations in diffusion properties of fiber tracts quantified by DTI and coherence measures quantified by MEG with respect to epileptogenicity possibly reflect the chronic microstructural cerebral changes associated with functional interictal activity. The proposed methodology for using MEG and DTI to investigate diffusion abnormalities related to focal epileptogenicity and propagation may provide a further means of noninvasive lateralization.














Similar content being viewed by others
References
Aghakhani Y, Liu X, Jette N, Wiebe S (2014) Epilepsy surgery in patients with bilateral temporal lobe seizures: a systematic review. Epilepsia 55:1892–1901
Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA (2013) Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: a systematic review and meta-analysis. Epilepsia 54:828–839
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851
Bagic AI, Knowlton RC, Rose DF, Ebersole JS, Committee ACPG (2011) American clinical magnetoencephalography society clinical practice guideline 1: recording and analysis of spontaneous cerebral activity*. J Clin Neurophysiol 28:348–354
Barkley GL, Baumgartner C (2003) MEG and EEG in epilepsy. J Clin Neurophysiol 20:163–178
Barron DS, Tandon N, Lancaster JL, Fox PT (2014) Thalamic structural connectivity in medial temporal lobe epilepsy. Epilepsia 55:e50–e55
Barron DS, Fox PT, Pardoe H, Lancaster J, Price LR, Blackmon K, Berry K, Cavazos JE, Kuzniecky R, Devinsky O, Thesen T (2015) Thalamic functional connectivity predicts seizure laterality in individual TLE patients: application of a biomarker development strategy. Neuroimage Clin 7:273–280
Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A (2004) Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. NeuroImage 23:717–723
Besson P, Dinkelacker V, Valabregue R, Thivard L, Leclerc X, Baulac M, Sammler D, Colliot O, Lehericy S, Samson S, Dupont S (2014) Structural connectivity differences in left and right temporal lobe epilepsy. Neuroimage 100:135–144
Bonilha L, Nesland T, Martz GU, Joseph JE, Spampinato MV, Edwards JC, Tabesh A (2012) Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 83:903–909
Brazier MA (1972) Spread of seizure discharges in epilepsy: anatomical and electrophysiological considerations. Exp Neurol 36:263–272
Bulacio JC, Jehi L, Wong C, Gonzalez-Martinez J, Kotagal P, Nair D, Najm I, Bingaman W (2012) Long-term seizure outcome after resective surgery in patients evaluated with intracranial electrodes. Epilepsia 53:1722–1730
Centeno M, Carmichael DW (2014) Network connectivity in epilepsy: resting state fMRI and EEG-fMRI contributions. Front Neurol 5:93
Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, Papademetris X, Fyson S, Lorincz M, Crunelli V (2009) DTI abnormalities in anterior corpus callosum of rats with spike–wave epilepsy. Neuroimage 47:459–466
Chiang S, Haneef Z (2014) Graph theory findings in the pathophysiology of temporal lobe epilepsy. Clin Neurophysiol 125:1295–1305
Cohen DJ, Hosaka JJ (1976) Magnetic field produced by a current dipole. J Electrocardiol 9:409–417
Concha L, Beaulieu C, Gross DW (2005) Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol 57:188–196
Concha L, Beaulieu C, Collins DL, Gross DW (2009) White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry 80:312–319
DeSalvo MN, Douw L, Tanaka N, Reinsberger C, Stufflebeam SM (2014) Altered structural connectome in temporal lobe epilepsy. Radiology 270:842–848
Dinkelacker V, Valabregue R, Thivard L, Lehericy S, Baulac M, Samson S, Dupont S (2015) Hippocampal-thalamic wiring in medial temporal lobe epilepsy: enhanced connectivity per hippocampal voxel. Epilepsia 56:1217–1226
Duda R, Hart P (1973) Linear Discriminant Functions. In: Classification P, Analysis S (eds) pp. Wiley-Interscience, New York, pp 130–185
Ebersole JS, Hawes-Ebersole SH (2007) Clinical application of dipole models in the localization of epileptiform activity. J Clin Neurophysiol 24:120–129
Elisevich K, Shukla N, Moran JE, Smith B, Schultz L, Mason K, Barkley GL, Tepley N, Gumenyuk V, Bowyer SM (2011) An assessment of MEG coherence imaging in the study of temporal lobe epilepsy. Epilepsia 52:1110–1119
Engel J Jr (1996) Surgery for seizures. N Engl J Med 334:647–653
England MJ, Liverman CT, Schultz AM, Strawbridge LM (2012) Epilepsy across the spectrum 25:266–276
Englot DJ, Hinkley LB, Kort NS, Imber BS, Mizuiri D, Honma SM, Findlay AM, Garrett C, Cheung PL, Mantle M, Tarapore PE, Knowlton RC, Chang EF, Kirsch HE, Nagarajan SS (2015a) Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain Res 138:2249–2262
Englot DJ, Nagarajan SS, Imber BS, Raygor KP, Honma SM, Mizuiri D, Mantle M, Knowlton RC, Kirsch HE, Chang EF (2015b) Epileptogenic zone localization using magnetoencephalography predicts seizure freedom in epilepsy surgery. Epilepsia 56:949–958
Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS (2008) Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 40:728–737
Gotman J (1981) Interhemispheric relations during bilateral spike-and-wave activity. Epilepsia 22:453–466
Govindan RM, Makki MI, Sundaram SK, Juhász C, Chugani HT (2008) Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res 80:30–41
Gross DW, Concha L, Beaulieu C (2006) Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 47:1360–1363
Hamalainen M, Hari R, Ilmoniemi J, Knuutila J, Lounamaa O (1993) Magnetoencephalography-theory, instrumentation and applications to noninvasive studies of the working human brain. Rev Mod Phys 65:413–497
Haneef Z, Lenartowicz A, Yeh HJ, Engel J Jr, Stern JM (2014a) Network analysis of the default mode network using functional connectivity MRI in Temporal Lobe Epilepsy. J Vis Exp. doi:10.3791/51442
Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J Jr, Stern JM (2014b) Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 55:137–145
He X, Doucet GE, Sperling M, Sharan A, Tracy JI (2015) Reduced thalamocortical functional connectivity in temporal lobe epilepsy. Epilepsia 56:1571–1579
Hosmer DW Jr, Lemeshow S, Sturdivant RX (2013) Applied logistic regression. Wiley, New York
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Keller SS, O’Muircheartaigh J, Traynor C, Towgood K, Barker GJ, Richardson MP (2014) Thalamotemporal impairment in temporal lobe epilepsy: a combined MRI analysis of structure, integrity, and connectivity. Epilepsia 55:306–315
Knowlton RC (2008) Can magnetoencephalography aid epilepsy surgery? Epilepsy Curr 8:1–5
Kohrman MH (2007) What is Epilepsy? Clinical perspectives in the diagnosis and treatment. J Clin Neurophysiol 24:87–95
Kraskov A (2004) Applications of synchronization and interdependence measures in particular to EEG of epilepsy patients. In: John Von Neumann Institute for Computing, NIC, vol. PhD thesis
Kuzniecky R, Bilir E, Gilliam F, Faught E, Palmer C, Morawetz R, Jackson G (1997) Multimodality MRI in mesial temporal sclerosis: relative sensitivity and specificity. Neurology 49:774–778
Lemkaddem A, Daducci A, Kunz N, Lazeyras F, Seeck M, Thiran JP, Vulliémoz S (2014) Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging. Neuroimage 5:349–358
Moran JE, Drake CL, Tepley N (2004) ICA methods for MEG imaging. Neurol Clin Neurophysiol 2004:72
Moran J, Bowyer S, Tepley N (2005) Multi-resolution FOCUSS: a source imaging technique applied to MEG data. Brain Topogr 18:1–17
Moran J, Manoharan A, Bowyer SM, Mason KM, Tepley N, Morrell M, Greene D, Smith BJ, Barkley GL (2006) MEG coherence imaging compared to electrocortical recordings from neuropace implants to determine the location of ictal onset in epilepsy patients. In: Cheyne D, Stroink BRG, Weinberg H (eds), 15th international conference on biomagnetism, vol 1300. Elsiver, Vancouver, pp 673–676
Morgan VL, Sonmezturk HH, Gore JC, Abou-Khalil B (2012) Lateralization of temporal lobe epilepsy using resting functional magnetic resonance imaging connectivity of hippocampal networks. Epilepsia 53:1628–1635
Mori S, van Zijl PC (2002) Fiber tracking: principles and strategies: a technical review. NMR Biomed 15:468–480
Nazem-Zadeh MR, Chapman CH, Lawrence TL, Tsien CI, Cao Y (2012) Radiation therapy effects on white matter fiber tracts of the limbic circuit. Med Phys 39:5603–5613
Nazem-Zadeh M-R, Elisevich KV, Schwalb JM, Bagher-Ebadian H, Mahmoudi F, Soltanian-Zadeh H (2014a) Lateralization of temporal lobe epilepsy by multimodal multinomial hippocampal response-driven models. J Neurol Sci 347:107–118
Nazem-Zadeh M-R, Schwalb JM, Elisevich KV, Bagher-Ebadian H, Hamidian H, Akhondi-Asl A-R, Jafari-Khouzani K, Soltanian-Zadeh H (2014b) Lateralization of temporal lobe epilepsy using a novel uncertainty analysis of MR diffusion in hippocampus, cingulum, and fornix, and hippocampal volume and FLAIR intensity. J Neurol Sci 342:152–161
Nazem-Zadeh M-R, Schwalb JM, Bagher-Ebadian H, Jafari-Khouzani K, Elisevich KV, Soltanian-Zadeh H (2014b) A Bayesian averaged response-driven multinomial model for lateralization of temporal lobe epilepsy. In: 2014 IEEE 11th international symposium on Biomedical Imaging (ISBI), pp 197–200
Nazem-Zadeh M-R, Elisevich K, Air EL, Schwalb JM, Divine G, Kaur M, Wasade VS, Mahmoudi F, Shokri S, Bagher-Ebadian H, Soltanian-Zadeh H (2015) DTI-based response-driven modeling of mTLE laterality. Neuroimage Clin
Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P (2001) Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13:1174–1185
Pittau F, Grova C, Moeller F, Dubeau F, Gotman J (2012) Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 53:1013–1023
Powell H, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, Duncan JS (2007) Abnormalities of language networks in temporal lobe epilepsy. Neuroimage 36:209–221
Rodrigo S, Oppenheim C, Chassoux F, Golestani N, Cointepas Y, Poupon C, Semah F, Mangin JF, Le Bihan D, Meder JF (2007) Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy Initial findings. Eur Radiol 17:1663–1668
Rugg-Gunn FJ (2007) Diffusion imaging in epilepsy. Expert Rev Neurother 7:1043–1054
Schevon CA, Cappell J, Emerson R, Isler J, Grieve P, Goodman R, McKhann JG, Weiner H, Doyle W, Kuzniecky R, Devinsky O, Gilliam F (2007) Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage 35:140–148
Schoffelen JM, Gross J (2009) Source connectivity analysis with MEG and EEG. Hum Brain Mapp 30:1857–1865
Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, Poldrack RA, Bilder RM, Toga AW (2008) Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 39:1064–1080
Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G (2008) Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage 43:554–561
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Song J, Tucker DM, Gilbert T, Hou J, Mattson C, Luu P, Holmes MD (2013) Methods for examining electrophysiological coherence in epileptic networks. Front Neurol 4:55
Sporns O (2011) The human connectome: a complex network. Ann N Y Acad Sci 1224:109–125
Stefan H, Nimsky C, Scheler G, Rampp S, Hopfengärtner R, Hammen T, Dörfler A, Blümcke I, Romstöck J (2007) Periventricular nodular heterotopia: a challenge for epilepsy surgery. Seizure 16:81–86
Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S (2001) Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage 14:723–735
Sutherling WW, Mamelak AN, Thyerlei D, Maleeva T, Minazad Y, Philpott L, Lopez N (2008) Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology 71:990–996
Thivard L, Adam C, Hasboun D, Clemenceau S, Dezamis E, Lehericy S, Dormont D, Chiras J, Baulac M, Dupont S (2006) Interictal diffusion MRI in partial epilepsies explored with intracerebral electrodes. Brain 129:375–385
Towle VL, Carder RK, Khorasani L, Lindberg D (1999) Electrocorticographic coherence patterns. J Clin Neurophysiol 16:528–547
Tufts DW, Kumaresan RIK (1982) Data adaptive signal estimation by singular value decomposition of a data matrix. Proc IEEE 70:684–685
Vaessen MJ, Jansen JF, Vlooswijk MC, Hofman PA, Majoie HM, Aldenkamp AP, Backes WH (2011) White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cereb Cortex 22:2139–2147 bhr298
Widjaja E, Simao G, Mahmoodabadi S, Ochi A, Snead O, Rutka J, Otsubo H (2010) Diffusion tensor imaging identifies changes in normal-appearing white matter within the epileptogenic zone in tuberous sclerosis complex. Epilepsy Res 89:246–253
Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC (1998) Automated image registration: i. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr 22:139–152
Xia M, Wang J, He Y (2013) BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8:e68910
Yogarajah M, Duncan JS (2007) Diffusion-based magnetic resonance imaging and tractography in epilepsy. Epilepsia 49:189–200
Yogarajah M, Powell HW, Parker GJ, Alexander DC, Thompson PJ, Symms MR, Boulby P, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, Duncan JS (2008) Tractography of the parahippocampal gyrus and material specific memory impairment in unilateral temporal lobe epilepsy. Neuroimage 40:1755–1764
Zalesky A, Fornito A, Bullmore ET (2010) Network-based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207
Zhang J, Liu Q, Mei S, Zhang X, Liu W, Chen H, Xia H, Zhou Z, Wang X, Li Y (2014) Identifying the affected hemisphere with a multimodal approach in MRI-positive or negative, unilateral or bilateral temporal lobe epilepsy. Neuropsychiatr Dis Treat 10:71
Acknowledgments
This work was supported in part by NIH grant R01-EB013227.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Andrew Zillgitt disclosures: Speaker’s Bureau for UCB and Lundbeck. Travel expenses paid by NeuroPace. None of other authors has any conflict of interest to disclose.
Appendix
Appendix
The following steps are taken for gray matter modeling:
-
1.
Create the AC-PC (anterior commissure- posterior commissure) coordinate system using the graphical interface to identify structural landmarks (Fig. 14).
-
2.
Identify the outline of the cortical surface on five MRI slices allowing the user to compensate for defects (Fig. 15). The slices are interpolated to a total number of 15 cortical slice boundaries.
-
3.
Using linear and locally nonlinear transforms a smooth cortical surface model is fit to the 15 cortical boundaries, including five user-drawn boundaries in Fig. 15 (Fig. 16).
Fig. 16 A smooth cortical surface model of the subject (in gray scale) is constructed to minimize the surface distance with 15 cortical boundaries. The cortical boundaries are depicted in blue, including five boundaries drawn by the user. The outer border of the gray matter in the cortical source model slices are depicted by red lines (Color figure online)
-
4.
The cortical gray matter is identified and a 4000 source location cortical model constructed for MEG imaging. The cortical surface model of the subject is adjusted to match the outer boundary of the cortical gray matter.
-
5.
The cortical surface is transformed to AC-PC coordinates. A combination of linear warps and shears is applied to the subject’s cortical surface to achieve the best match to a cortical surface model of the MNI305 brain. The shears align the anterior and posterior poles of the subject cortical surface with the MNI surface model. A closest neighbor algorithm is used to identify the corresponding subject and MNI surface points which are used in all transform calculations. These transforms operate on the volume within the cortical surface as well as the surface itself.
-
6.
Within a sequence of three to five overlapping Gaussian windows, second order transforms of included brain volume are calculated along the inferior-superior axis (Z) first, left–right axis (X) second and posterior-anterior (Y) axis last (Fig. 17). These locally nonlinear brain volume transforms further optimize the match of the subject cortical surface to the MNI surface model within each window (Fig. 18), using the following equation:
Fig. 17 Five overlapped window functions are shown along the posterior-anterior axis of the cortical surface. The maximum amplitude of each window function is 1. Adjacent window functions overlap and the sum of their amplitudes is one. Thus, the full transform throughout the cortical volume is a continuous mixture of windowed transforms. The same windowing technique is applied along the inferior-superior axis and left–right axis. Furthermore, these one dimensional windows can be altered to be two- or three-dimensional windows to achieve more focal sensitivity to mismatch and to accommodate internal structural matching (Color figure online)
where A is transform matrix, XYZ, and \({\text{X}}_{\text{MNI}} {\text{Y}}_{\text{MNI}} {\text{Z}}_{\text{MNI}}\) are the native and MNI coordinate systems, respectively. The MNI coordinate system specifies the location of the brain structure within an AC-PC coordinate system (Fig. 14). Generalized Gaussian window functions are used to eliminate transform discontinuities. The inverse transform is generated to convert MRI to MNI305 coordinates. The algorithm generates a set of sequentially applied transforms that are applied to the original MRI pixel coordinates of the MEG imaging results.
Rights and permissions
About this article
Cite this article
Nazem-Zadeh, MR., Bowyer, S.M., Moran, J.E. et al. MEG Coherence and DTI Connectivity in mTLE. Brain Topogr 29, 598–622 (2016). https://doi.org/10.1007/s10548-016-0488-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-016-0488-0