Abstract
Objective
To investigate the neural correlates of working memory during a spontaneous migraine attack compared to the interictal phase, using functional magnetic resonance imaging (fMRI).
Background
Cognitive disturbances are commonly observed during migraine attacks, particularly in the headache phase. However, the neural basis of these changes remains unknown.
Methods
In a fMRI within-subject test-retest design study, eleven women (32 years of age, average) with episodic migraine were evaluated twice, first during a spontaneous migraine attack, and again in a pain-free period. Each session consisted in a cognitive assessment and fMRI while performing a working memory task (N-back).
Results
Cognitive test scores were lower during the ictal session than in the pain-free session. Regions typically associated with working memory were activated during the N-back task in both sessions. A voxel wise between session comparison showed significantly greater activation in the left frontal pole and orbitofrontal cortex during the attack relative to the interictal phase.
Conclusion
Migraine patients exhibited greater activation of the left frontal pole and orbitofrontal cortex while executing a verbal working memory task during a spontaneous migraine attack when compared to the interictal state. Given the association of these regions with pain processing and inhibitory control, these findings suggest that patients recruit inhibitory areas to accomplish the cognitive task during migraine attacks, a neural signature of their cognitive difficulties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a prevalent neurological disorder that affects approximately an 11.6% of the global population [1], and according to the Global of Burden Disease study 2019, it is the second cause of neurological disability in young and middle-aged adults [2]. Migraine is characterized by a cyclical and episodic pattern of recurrent attacks (ictal phase) of severe headaches and other associated symptoms that include reversible cognitive disturbances [3, 4]. These cognitive difficulties are reported by almost 40% of patients and consist in attention and word retrieval difficulties, executive dysfunction with impairments in multitasking, inhibitory control, attention shifting and working memory [5, 6]. To date, few studies have objectively analysed the nature of cognitive disturbances during spontaneous migraine attacks. Two of such studies used a within-subject design, wherein migraine participants were assessed twice, during the ictal phase and during a pain-free period (interictal phase) and found a consistent decline in performance during the ictal phase, which was significant for cognitive screening tests and executive function tasks [7, 8]. Although pain may contribute to these difficulties, it cannot completely explain the cognitive decline since cognitive complaints often begin before the onset of headache, in the preictal phase [9].
Studies using resting-state functional Magnetic Resonance Imaging (fMRI) have shed light on the complex interaction between cognitive and pain processing areas during pain stimulation and spontaneous migraine attacks. In one study, participants with migraine were scanned during a spontaneous attack and compared to a control population revealing a change in connectivity between cognitive-related networks, specifically the Executive Control Network and the Dorsal - Ventral Attention System, as well as abnormal connectivity between the middle frontal gyrus and the insula [10, 11]. Another study, employing positron emission tomography, found consistent activation of the insula and prefrontal cortex, among other areas, during the first 24 h of spontaneous migraine attacks, when compared to the same cohort’s interictal phase [12]. A report exploring attentional networks using task-fMRI under induced pain condition, observed distinct patterns of neural cognitive-pain interaction in migraine compared to controls including deactivation of the dorsolateral prefrontal cortex and left dorsal anterior midcingulate cortex [13]. The reported findings observed effects of pain or spontaneous migraine attack on brain function despite their small samples of 5 to 20 volunteers [10,11,12,13]. These studies report on heterogeneous samples, including both cases with and without aura [12], chronic as well as episodic migraine [13], or compared different cohorts of cases in the ictal phase to controls [10, 11].
To the best of our knowledge, the patterns of brain activity while performing cognitive tasks, have not been studied during spontaneous migraine attacks. The aim of our study was to tackle the changes of neural resources subserving working memory during spontaneous migraine attacks and compare them to a retest assessment conducted in pain-free periods within the same cohort. To assess this cognitive domain comprehensively, we also evaluated cognitive performance with a brief neuropsychological battery. Based on previous findings and using a similarly sized sample [10,11,12,13], we hypothesized to observe a decline in N-back performance accompanied by activation in pain related areas during the ictal phase when compared to the interictal phase.
Methods
We conducted a prospective test-retest within-subject design study including neuropsychological evaluation and fMRI acquisition. This study is part of a larger research project that also included perfusion evaluation using arterial spin labelling, which results have been published elsewhere [14]. The study protocol and statistical analysis were not preregistered. The neuroimaging and neuropsychological assessment protocols and patients consent form were reviewed and approved by the Hospital da Luz Ethics Committee.
Population
Adults diagnosed with episodic migraine without aura according to the International Headache Society criteria [15] (ICHD 2018) were recruited during a medical appointment at the Headache Outpatient Clinic of Hospital da Luz. Additional inclusion criteria required right handedness and absence of prophylactic migraine medication at inclusion. Exclusion criteria consisted in the presence of psychiatric disorders, psychiatric medication or the presence of neurological diseases other than migraine. All participants signed the informed consent.
Procedure
Clinical and sociodemographic data were collected at the recruitment session through a structured clinical interview by a neurologist, including age of migraine onset and current attack frequency, duration and intensity. Migraine disability was assessed through the headache impact test (HIT-6) [16].
The study protocol consisted in two sessions, the first of which was conducted during a spontaneous migraine attack (ictal session, S-ictal). For this session, patients were instructed to contact the study team by phone at the beginning of an attack if they were experiencing a minimal pain intensity of 4 in a 0–10 of the visual analogue scale (VAS). Once the availability of the scanner was confirmed, patients were invited to the MRI department and instructed to abstain from taking acute migraine medication up to 12 h before the scan. Attack-associated symptoms other than pain were recorded before the exam.
The second session took place at least 1 month after the first session, during an interictal phase (S-interictal), defined as being headache free for at least 48 h before and after the scan session, which was confirmed by a telephone contact 72 h after that session.
In both sessions, a brief neuropsychological evaluation and screening of depressive symptoms with the Beck Depression Inventory (BDI) [17] was performed additionally to the scan. Session order was fixed, ictal first, followed by interictal.
Neuropsychological battery
We used a neuropsychological battery that had been previously applied to migraine patients. It is focused on attention and executive functions [18], including the following tests: Stroop Test [19] as a measure of inhibitory control; Trail Making Test B for alternate attention and shifting ability; a Phonological Verbal Fluency task (with letters P and M), for verbal initiative and monitoring; Finger Tapping [20] to measure motor speed; Trail Making Test A [21] and Symbol Digit from the Wechsler Adult Intelligence Scale-III (WAIS-III) [22] for sustained attention and visual processing speed.
Image acquisition
MRI data was obtained on a 3 Tesla Siemens Verio Systems scanner with a 12-channel radiofrequency head coil. In both sessions, a Blood Oxygen Level Dependent (BOLD) fMRI acquisition was performed using a gradient echo-planar imaging (GE-EPI) pulse sequence (TR/TE = 2000/30 ms, voxel size = 4.0 × 4.0 × 3.6 mm3, number of slices = 22, number of volumes = 210). Anatomical images were collected using a T1-weighted sequence (Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE) TR/TE = 2250/2.26 ms, voxel size = 1 × 1 × 1 mm3).
N-back task
A block design of the verbal N-back task with 2 conditions (0-back and 2-back) was administered using Nordic NeuroLab hardware and goggles (www.nordicneurolab.com). The N-back paradigm was originally developed by Gevins and Cutillo in 1993 [23] to assess working memory using electrophysiology measures and was subsequently adapted for fMRI a year later [24]. It has been widely used ever since because it requires the updating and manipulating of remembered information, placing different levels of demand on working memory [25]. In the verbal N-back, participants are required to monitor a series of letters shown on the screen and to respond whenever a target letter appears. The 0-back condition was used to access selective attention and vigilance networks, where the target is every appearance of a randomized pre-selected letter in the letter sequence. In the 2-back condition, the target letter is defined as any letter that is identical to the one presented two positions previously in the sequence. By combining both conditions, 0-back as baseline and 2-back for working memory, brain regions related to the active maintenance of information about the stimuli were identified23. Each block lasted for 42 s, with a sequence of 21 letters that was presented in a pseudorandomized order for 1 s each, with a 1-s interstimulus interval. Five blocks of conditions 0-back and five of 2-back were presented, with a total duration of 420 s or 7 min. Hit and incorrect response rates are used as behavioural measures of the task.
Data analysis
The fMRI data was analysed using FSL, the FMRIB Software Library (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Pre-processing consisted of motion correction, fieldmap distortion correction, high-pass temporal filtering (frequency cutoff = 100 s) and spatial smoothing (Gaussian kernel with FWHM = 5 mm). Functional images were registered into the high-resolution anatomical images of the same patient, which were in turn registered to the Montreal Neurological Institute (MNI) standard space using nonlinear registration. First-level statistical analysis was performed using a general linear model (GLM) including two explanatory variables (EVs) generated based on boxcar functions corresponding to the presentation of the 0-back and 2-back stimulus blocks, each convolved with a double-gamma hemodynamic response function. Moreover, standard motion parameters were also included in the GLM as confound EVs. The contrast 2-back > 0-back was defined to assess brain activation associated with working memory. Group analysis of this contrast was performed voxel wise for the whole brain, using the well-established methodology implemented in the FSL tool FEAT/FLAME1 [26], this time building a GLM for repeated measures analysis and testing for the average effect across sessions as well as for differences between sessions [27]. Correction for multiple comparisons was performed using a cluster significance threshold of p < 0.05 (and an initial voxel z > 2.3) This procedure is consistent with the one used in previous studies [28, 29].
We further investigated whether the activity of the brain region exhibiting differences between phases is related to the neuropsychological and clinical scores of the patients. For this purpose, we defined a region of interest (ROI) based on the cluster exhibiting significant differences between phases (S-ictal > S-interictal) obtained in the whole brain group analysis We then used FSL’s tool Featquery to compute the mean BOLD signal change in this ROI, for each patient and session.
IBM SPSS Statistics 28 version was then used for statistical analysis. Given the sample size (N = 11) we used nonparametric measures, and no statistical power calculation was conducted. We applied two-tailed Spearman correlations between disease duration and attack frequency, the seven clinical parameters of the ongoing migraine attack: pain duration in hours and the intensity of pain, nausea, photo and phonophobia, movement intolerance and difficulties in concentration (rated in VAS scale), N-back performance (accuracy and incorrect %) and the scores obtained in the HIT-6 and BDI questionnaires. All the above with the main ROI analysis result using a significance level of p < 0.005 applying Bonferroni correction for 14 comparisons. For neuropsychological and N-back test-retest data, the Wilcoxon test for related samples was used, and statistical significance was set at p < 0.05 and adjusted for multiple comparisons using the Signed Rank test from SPSS software.
Results
Population
Fourteen women with episodic migraine without aura were recruited, and eleven completed the protocol. Two participants did not complete both sessions of the protocol and another participant lacked the behavioural data of the N-back, recorded in the second session, due to technical difficulties. Table 1 provides clinical and sociodemographic data from the final sample of 11 participants. Symptoms at S-ictal are also presented. The HIT-6 questionnaire had a median score of 52, interpreted as experiencing a moderate impact of migraine.
Neuropsychological assessment
All neuropsychological tasks showed, on average, a lower performance in S-ictal, the first session, compared to the S-interictal (results presented in Table 2). Subjects were slower in both TMT A and TMT B and had lower scores in cognitive flexibility, processing speed (letter fluency, digit symbol), with a lower number of words named and symbols drawn respectively. This difference was significant in Stroop test’s interference score (p = 0.010) and in the finger tapping of the left hand (p = 0.006). There was no significant difference in depressive symptoms between sessions (p = 0.289). The average BDI score was 7.3, indicating “no depression”, and was higher during S-ictal compared to S-interictal (9.9 vs 4.7 points, p = 0.289). Performance on the 2-back task increased from an average hit rate of 87% during the attack, to a 92% in the interictal session, which was not significant.
Behavioural measures did not correlate with the time between assessments in the 2-back (Spearman r = .18; p = 0.600), nor the in Finger Tap left (r = 0.18; p = 0.601) or Stroop (r = .09; p = 0.790).
fMRI
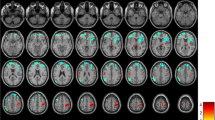
Group mean maps of activation during the N-back (contrast 2-back > 0-back) showed significant activity in areas relevant to verbal working memory, including the lateral occipital cortex, parietal lobule and insula. There was an overall higher activation in the first session (S-ictal) compared to S-interictal (Fig. 1 and Table 3).
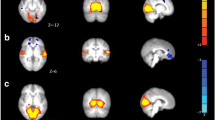
In the analysis of S-ictal > S-interictal, significantly higher activation was found in the frontal lobe, including regions of the left frontal pole and the orbitofrontal cortex (Fig. 2 and Table 4). No significantly higher activation was found for S-interictal > S-ictal. This cluster of activation was subsequently utilized as ROI to compare its level of activation for each patient and session with different clinical and neuropsychological variables.
The average percentage of BOLD signal change during N-back in the ROI is shown for each session and patient in Fig. 3. Consistently with the group results, most patients showed activity in this ROI during S-ictal, which was diminished or even deactivated during S-interictal (Fig. 3).
The activation of this ROI in the left frontal pole and orbitofrontal cortex during the migraine attack did not correlate with the N-back performance in accuracy (r = − .34; p = 0.304) nor incorrect responses (r = .50; p = 0.115). It neither correlated with the BDI questionnaires (r = .324; p = 0.331) nor the HIT-6 scores where only a trend was observed (r = .71; p = 0.014). Neither did we find significant correlations between the percentage BOLD signal change in the ROI and clinical features of disease duration (r = .17; p = 0.619), attack frequency (r = .10; p = 0.779), nor with the studied migraine attack clinical parameters, namely: pain duration (r = − .25; p = 0.467), pain severity (r = − .28; p = 0.412), nausea (r = − .36; p = 0.275), photophobia (r = − .33; p = 0.322), phonophobia (r = .01; p = 0.989), worsening with movement (r = − .27; p = 0.414) and concentration difficulties (r = 0.11; p = 0.748).
Discussion
In a prospective within-subject test-retest study, we investigated working memory in migraine using neuropsychological assessment and a cognitive task with fMRI, during and between spontaneous migraine attacks. We found that patients obtained comparatively lower cognitive scores during migraine attacks and also that performance in a working memory task during the attack increased the activation of areas related to inhibitory control and pain. To our knowledge, this is the first study documenting the neural correlates of a demanding executive task performed during migraine attacks, a finding that contributes to explain patients’ complaints.
Neuropsychological assessment
Cognitive scores of both sessions were within expected ranges based on age and education. However, an overall enhancement in neuropsychological performance was evident during the second, S-interictal assessment, compared to S-ictal, which was significant in the Stroop test and non-dominant finger tapping. A comparable trend of improvement has been noted in a test-retest study using the same battery in healthy controls and interictal migraine participants, showing a significant difference only in the Stroop test17. These observed improvements may be attributed to the learning effect on the second session. While the time interval between evaluations was longer in the earlier study (80 days), the absolute enhancement observed here surpassed what was observed in the previous data. This implies that the absence of pain could have influenced the magnitude of the expected learning effect, in our sample.
fMRI
The improvement in the N-back task performance and the reduced brain activity that were observed in the second session, although not correlated, could be explained either by a learning effect or a decline in mental effort, associated to the absence of pain. A study using a 2-back task in healthy individuals with a shorter test-retest interval (14.6 days), observed a decrease in functional activity in the parietal and dorsolateral prefrontal cortex between first and second session, which was attributed to the learning effect [30]. The observed improvement in performance that increased from 93.2 to 95.5% of hit accuracy in that study was not statistically significant and less expressive than what was found in our data. The higher magnitude of difference observed in our participants suggests that there was an additional effect of the migraine attack affecting participants’ performance in the first session. This finding supports the hypothesis that pain impairs working memory function, as suggested by a report of a negative relationship between pain severity and N-back behavioural measures in subjects with diverse pain conditions [31]. Altogether, this data indicates the cost of sharing limited attentional resources between cognitive functions and pain.
In fact, we identified a cluster on the left orbital prefrontal cortex that was significantly more activated in the ictal session during the N-back paradigm. This area is not generally considered to be part of the working memory network, regardless of the modality (auditory or visual) in which the cognitive effort is generated [32]. For verbal working memory, namely with the letter N-back, areas activated include the middle frontal gyrus and parietal lobe regions [33]. Conversely, the orbito-prefrontal cortex is relevant for inhibition, impulse control and decision-making [34]. However, it is also observed under trigeminal pain stimulation and ictal phase, along with activation of somatosensory cortices and other brain areas indicating that it can be related to pain processing [35]. Earlier research has also reported high functional connectivity between regions associated with pain and sensory processing, such as the right thalamus and contralateral pain processing regions, including the orbitofrontal cortex [36]. In a similar study which also compared spontaneous attacks and interictal phase in migraine without aura but not during a cognitive task, significant functional connectivity between the insula and the frontal pole was noted during painful stimulation in patients with high frequency of attacks [37].
Alternatively, the higher activation of the left orbital prefrontal cortex observed in the ictal phase could suggest that patients are employing greater inhibitory control to perform the task.
No significant correlation was identified between the prefrontal cortex activation during S-ictal and N-back performance, depression questionnaire, disease duration, attack frequency or the clinical features of the studied attack. However, individuals reporting higher disability (according to the HIT-6) displayed higher brain activity in that region during the attack, although statistical significance was not achieved. A preceding study investigating resting state in interictal migraine without aura established an inverse correlation between the HIT-6 score and impaired connectivity in the somatosensory cortex [38].
Taken together, these findings reinforce the concept that activity in brain regions associated to pain processing might influence the neural correlates of cognitive performance during migraine attacks.
Limitations
In an ideal study design, the migraine phases would be balanced, and session repetition should be randomized to minimize the learning bias. However, for practical recruitment issues and to improve effective participation, the researchers prioritized ictal sessions. A similar design was used in a study which reported difficulties alternating the sessions while studying brain activity during spontaneous migraine attacks [39]. Having a healthy control group would be necessary to compare the test-retest learning and familiarity effect of the task and the context of the fMRI exam. The sample size of eleven exclusively female participants limits the generalizability of the findings. Another relevant limitation is that the scans were made in different time frames within the attack, and there is evidence that brain activation may differ according to the timing from the onset [40].
Conclusion
The present study used fMRI to investigate the neural basis of working memory during spontaneous migraine attacks. Our results showed a decrease in performance during ictal phase compared to interictal, although scores of both sessions were within expected ranges based on age and education. We also observed significant prefrontal activation during the ictal phase indicating a potential interaction between the pain network and areas involved in cognitive processing. While further research is necessary to fully understand the impact of migraine attacks on cognitive functioning, our findings support the existence of a neural basis for cognitive complaints reported by migraine patients during attacks.
Data availability
The data that supports the findings of this study are available from the authors upon reasonable request.
References
Woldeamanuel YW, Cowan RP (2017) Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci 372:307–315. https://doi.org/10.1016/j.jns.2016.11.071
Feigin VL, Nichols E, Alam T et al (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Gil-Gouveia R, Martins IP (2018) Clinical description of attack-related cognitive symptoms in migraine: a systematic review. Cephalalgia 38(7):1335–1350. https://doi.org/10.1177/03331024177282
Vuralli D, Ayata C, Bolay H (2018) Cognitive dysfunction and migraine. J headache pain 19(1):1–4. https://doi.org/10.1186/s10194-018-0933-4
Gil-Gouveia R, Oliveira AG, Martins IP (2015) Assessment of cognitive dysfunction during migraine attacks: a systematic review. J Neurol 262(3):654–665. https://doi.org/10.1007/s00415-014-7603-5
Gil-Gouveia R, Oliveira AG, Martins IP (2016) Subjective cognitive symptoms during a migraine attack: a prospective study of a clinic-based sample. Pain Phys 19(1):137–150
Meyer JS, Thornby J, CrawfordK RGM (2000) Reversible cognitive decline accompanies migraine and cluster headaches. Headache: The Journal of Head and Face. Pain 40(8):638–646. https://doi.org/10.1046/j.1526-4610.2000.040008638.x
Gil-Gouveia R, Oliveira AG, Martins IP (2015) Cognitive dysfunction during migraine attacks: a study on migraine without aura. Cephalalgia 35(8):662–674. https://doi.org/10.1177/0333102414553823
Giffin NJ, Ruggiero L, Lipton RB, Silberstein SD, Tvedskov JF, Olesen J, Altman J, Goadsby PJ, Macrae A (2003) Premonitory symptoms in migraine: an electronic diary study. Neurol. https://doi.org/10.1177/0333102414553823
Coppola G, Di Renzo A, Tinelli E et al (2016) Thalamo-cortical network activity during spontaneous migraine attacks. Neurol 87(20):2154–2160. https://doi.org/10.1212/WNL.0000000000003327
Coppola G, Di Renzo A, Tinelli E et al (2018) Resting state connectivity between default mode network and insula encodes acute migraine headache. Cephalalgia 38(5):846–854. https://doi.org/10.1177/0333102417715230
Afridi SK, Giffin NJ, Kaube H et al (2005) A positron emission tomographic study in spontaneous migraine. Arch Neurol 62(8):1270–1275. https://doi.org/10.1001/archneur.62.8.1270
Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, Seminowicz DA (2015) Altered cognition-related brain activity and interactions with acute pain in migraine. NeuroImage: Clin 7:347–358. https://doi.org/10.1016/j.nicl.2015.01.003
Gil-Gouveia R, Pinto J, Figueiredo P, Vilela PF, Martins IP (2017) An arterial spin labeling MRI perfusion study of migraine without aura attacks. Front neurol 8:280. https://doi.org/10.3389/fneur.2017.00280
Arnold M (2018) Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia 38:1–211. https://doi.org/10.1177/033310241773820
Constantino MF (2005) Adaptação e Validação Intercultural do Headache Impact Test – 6 Item (HIT-6). Dissertation. Faculdade de Motricidade Humana da Universidade Técnica de Lisboa, Portugal
Beck AT, Steer RA, Brown GK (1996) BDI-II: Beck depressioninventory. Pearson
Gil-Gouveia R, Oliveira AG, Martins IP (2016) Sequential brief neuropsychological evaluation of migraineurs is identical to controls. Acta Neurol Scand 134:197–204. https://doi.org/10.1111/ane.12530
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18(6):643–662. https://doi.org/10.1037/h0054651
Lezak MD, Howieson DB, Loring DW, Fischer JS (2004) Neuropsychological assessment. Oxford University Press, USA
Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Percept motor skills 8:271–276
Wechsler D (1997) Wechsler adult intelligence scale-III. The Psychological Corporation, San Antonio
Gevins A, Cutillo B (1993) Spatiotemporal dynamics of component processes in human working memory. Electroencephal Clin Neurophys 87:128–143. https://doi.org/10.1016/0013-4694(93)90119-G
Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Mickleborougholl N (1994) Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. HBM 1:293–304. https://doi.org/10.1002/hbm.460010407
Cohen JD, Perlstein WM, Braver TS et al (1997) Temporal dynamics of brain activation during a working memory task. Nature 386(6625):604–608. https://doi.org/10.1038/386604a0
Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM (2004) Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21:1732–1747. https://doi.org/10.1016/j.neuroimage.2003.12.023
Beckmann CF, Jenkinson M, Smith SM (2003) General multilevel linear modelling for group analysis in FMRI. Neuroimage 20(2):1052–1063. https://doi.org/10.1016/S1053-8119(03)00435-X
Parry AM, Scott RB, Palace J, Smith S, Matthews PM (2003) Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain 126(12):2750–2760. https://doi.org/10.1093/brain/awg284
Osterbauer RA, Matthews PM, Jenkinson M, Beckmann CF, Hansen PC, Calvert GA (2006) Color of scents: chromatic stimuli modulate odor responses in the human brain. Chem Senses 31(6):531–538. https://doi.org/10.1152/jn.00555.2004
Plichta MM, Schwarz AJ, Grimm O et al (2012) Test–retest reliability of evoked BOLD signals from a cognitive–emotive fMRI test battery. Neuroimage 60:1746–1758. https://doi.org/10.1016/j.neuroimage.2012.01.129
Attridge N, Noonan D, Eccleston C, Keogh E (2015) The disruptive effects of pain on n-back task performance in a large general population sample. Pain 156:1885–1891. https://doi.org/10.1097/j.pain.0000000000000245
Baddeley A (2003) Working memory: looking back and looking forward. Nat rev neurosci 4:829–839. https://doi.org/10.1038/nrn1201
Owen AM, McMillan KM, Laird AR, Bullmore E (2005) N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. HBM 25:46–59. https://doi.org/10.1002/hbm.20131
Bechara A, Damasio H, Damasio AR (2000) Emotion, decision making and the orbitofrontal cortex. Cerebral cortex 10:295–307. https://doi.org/10.1093/cercor/10.3.295
Schwedt TJ, Chiang CC, Chong CD, Dodick DW (2015) Functional MRI of migraine. Lancet Neurol 14:81–91. https://doi.org/10.1016/S1474-4422(14)70193-0
Amin FM, Hougaard A, Magon S et al (2018) Altered thalamic connectivity during spontaneous attacks of migraine without aura: a resting-state fMRI study. Cephalalgia 38:1237–1244. https://doi.org/10.1177/0333102417729113
Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D (2012) Concurrent functional and structural cortical alterations in migraine. Cephalalgia 32:607–620
Zhang J, Su J, Wang M et al (2017) The sensorimotor network dysfunction in migraineurs without aura: a resting-state fMRI study. J Neurol 264:654–663. https://doi.org/10.1007/s00415-017-8404-4
Denuelle M, Fabre N, Payoux P et al (2007) Hypothalamic activation in spontaneous migraine attacks. Headache 47:1418–1426. https://doi.org/10.1111/j.1526-4610.2007.00776.x
Schulte LH, May A (2016) The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 139:1987–1993. https://doi.org/10.1093/brain/aww097
Acknowledgements
We extend our gratitude to the participants for their time and cooperation. Our sincere appreciation also goes to the administrative staff and neuroimaging technicians at Hospital da Luz for their invaluable assistance.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethic approval
The study conformed to the principles of the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Hospital da Luz - Lisboa. Informed consent All participants were informed about the experimental procedures and gave their informed written consent to participate in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruiz-Tagle, ., Figueiredo, P., Pinto, J. et al. Working memory during spontaneous migraine attacks: an fMRI study. Neurol Sci 45, 1201–1208 (2024). https://doi.org/10.1007/s10072-023-07120-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07120-0