Abstract
Introduction
The COVID-19 pandemic led to psychological consequences on people’s mental health, representing a condition of increased vulnerability for the weakest sections of population, including elderly patients with Parkinson’s disease (PD).
This longitudinal study aimed at exploring the impact of the most frequent non-motor symptoms and their contribute on health-related quality of life of PD patients after the COVID-19 outbreak, in comparison with the pre-pandemic status.
Methods
Forty-two non-demented PD patients underwent a first assessment between December 2018 and January 2020 (T0). Then, between March and May 2021 (T1), they were contacted again and asked to complete the second assessment. Levels of global functioning, several non-motor symptoms (i.e. depression, apathy, anxiety, anhedonia) and health-related quality of life were investigated.
Results
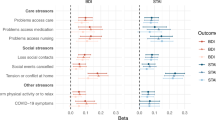
Results of the the paired Wilcoxon signed-rank test showed that at T1, PD patients scored lower on the emotional subscale of the DAS, Z = − 2.49; p = 0.013; Cohen dz = 0.691. Higher scores of the TEPS total score, Z = − 2.38; p = 0.025; Cohen dz = 0.621, and LEDD, Z = − 2.63; p = 0.008; Cohen dz = 0.731, were also reported at T1.
Conclusion
The present study suggested that self-isolation at home might lead to a reduction of apathy and anhedonia in PD patients due to the increase in social support provided by families during COVID-19 restrictions. This evidence brings out the need of a consistent and persistent social support which might be represented by caregivers or/and social assistive robotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronavirus disease 2019 (COVID-19) has greatly impacted people’s mental health, causing severe psychological consequences. Indeed, people experienced negative emotions (i.e. depressive symptoms, anxiety, emotional exhaustion, anger, trauma-related mental health disorders, insomnia) and negative cognitive assessment for self-protection such as fear of falling sick or dying and decreasing perception of wellbeing [1].
Particular groups appeared to be at higher risk for this kind of mental health impact, for instance the elderly, children, college students, homeless individuals and those in economic vulnerability and psychiatric patients [1]. Following the abovementioned findings, increased attention has been paid to the putative more severe consequences experienced by people with chronic neurologic diseases, such as patients with Parkinson’s disease (PD). In fact, some studies found that many PD patients are concerned about COVID-19 risk [2] and reported higher levels of stress and anxiety during the pandemic [3].
In addition, lockdown measures to mitigate the spread of COVID-19 deeply changed people’s daily lives in the short-term. According to Helmich and colleagues [4] successfully coping with such a sudden change requires cognitive operations depending on the normal functioning of dopaminergic structures and it is also well-known the link between impaired dopaminergic signalling and psychological post-traumatic stress [5].
Nigro-striatal dopamine depletion is the most relevant pathophysiological process in PD and, therefore, PD patients may be particularly vulnerable to negative psychological consequences following the COVID-19 pandemic and lockdown measures. Few cross-sectional surveys already provided insights about the greater vulnerability of PD patients to experience COVID-19 outbreak-related stress [2]. Another study [6] further explored whether pre-lockdown clinical features may be associated with the psychological impact of COVID-19 quarantine revealing that the psychological impact of a 40-day quarantine was associated with pre-lockdown levels of anxiety, treatment-related motor complications, quality of life and lockdown hours per day. However, until now, no longitudinal study systematically explored whether COVID-19 outbreak caused an aggravation of pre-existent non-motor symptoms and/or a deterioration in quality of life after the pandemic. Indeed, it is widely known that non-motor symptoms, such as apathy, anxiety and reduced functional autonomy are frequently reported in PD patient and can profoundly impact on their quality of life [7].
Taking into account the abovementioned reports, we aimed to explore the putative worsening of the most frequent non-motor symptoms in PD after the COVID-19 pandemic, by means of symptom-specific scales.
Materials and methods
Participants
For the present study, 68 PD outpatients were recruited from the Institute of Diagnosis and Care “Hermitage” of Naples (Italy). Participants underwent the first assessment between December 2018 and January 2020 (T0), before the COVID-19 outbreak. Then, between March and May 2021 (T1), they were contacted again and asked to complete the second assessment: 25 out of 68 participants did not give their consent for lack of interest or no time, so the final sample was of 42 PD patients. T1 neuropsychological assessment was conducted through telephone interview and demographic features (i.e. gender, age, years of schooling) were recorded. Clinical aspects (i.e. disease duration, Levodopa Equivalent Daily Dose, LEDD, severity of motor symptoms assessed by both part III of UPDRS and Hoehn and Yahr staging, H&Y) were recorded in person by neurologists during routine exams at the clinical facility. To be included in the study, each PD patient had to be without a clinical diagnosis of dementia or cognitive impairment (defined as total age-and education-adjusted score ≥ 15.5 at Montreal Cognitive Assesment (MoCA [8]) and with no clinical diagnosis of major concurrent neurological disorders. Furthermore, for each PD patient, severity of motor symptoms and psychiatric therapy must have been stable between T0 and T1 and they must not have undergone any kind of rehabilitation interventions (e.g. use of neuromodulation techniques such as Transcranial Direct Current Stimulation) from T0 to T1.
The research was conducted after PD patients provided their consent approved by the Local Ethics Committee and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Socio-demographic aspects, such as age and education level, were recorded. Moreover, before starting the second assessment at T1, each of participants was asked if they had contracted the COVID-19 virus.
Measures
The Montreal Cognitive Assessment (MoCA [8]) assessing global functioning was administered at T0. Moreover, all PD patients completed self-report questionnaires assessing some of the most frequent non-motor symptoms both at T0 and at T1.
Anxiety was assessed by the Parkinson Anxiety Scale (PAS [9]), a self-report scale composed by 12-items rated on a 5-point Likert scale, with 0 representing “not or never” and 4 representing “severe or almost always”, divided into three subscales: persistent anxiety (5 items), episodic anxiety (4 items) and avoidance behaviour (3 items). The total score range is from 0 to 48, with higher scores indicating more severe anxiety (cut-off score ≥ 9).
Depression was assessed by the Beck Depression Inventory II (BDI-II [10]), a 21-item self-report inventory measuring the severity of depression. The score range is from 0 to 63, with higher scores indicating more severe depressive symptoms.
Apathy was assessed by the Dimensional Apathy Scale (DAS [11]), a self-report questionnaire composed by 24-items rated on a 4-point Likert scale, with 0 representing “almost always” and 4 representing “almost never”. The total score range is from 0 to 72, with higher score indicating more severe apathy.
The presence of anhedonia was assessed by the Temporal Experience of Pleasure Scale (TEPS [12]), a self-report scale evaluating two hedonic components: anticipatory pleasure (10 items) and consummatory pleasure (8 items). It consists of 18 items rated on a 6-point Likert scale, with 1 representing “very false” and 6 “very true”. The score range is from 18 to 108, with lower scores indicating the presence of anhedonia.
The Parkinson’s Disease Quality of Life Questionnaire (PDQ-8 [13]) was used to measure self-perceived health and health-related quality of life (HRQoL). It consists of 8-items that refers to how patients have experienced difficulties due to PD in the preceding month, rated on a 5-point Likert scale, with 0 representing “never” and 4 representing “always”. The total score ranges from 0 to 100, with lower scores indicated greater QoL levels.
Statistical analysis
Preliminary descriptive analyses on demographic (i.e. gender, age, years of schooling), clinical (i.e. disease duration, Levodopa Equivalent Daily Dose, UPDRS, H&Y) and cognitive (MoCA) features at T0 were executed. The percentage of PD patients which contracted the COVID-19 virus at T1 in was also computed. Since there were several significant violations of assumption of normality (Shapiro–Wilk p-value < 0.05), non-parametric statistics were conducted. The paired Wilcoxon signed-rank test was conducted on clinical (i.e. LEDD, UPDRS-III, H&Y) and behavioural (i.e. PAS, BDI-II, DAS, TEPS, PDQ-8 scores and sub-scores when provided) features to investigate differences between T0 and T1. Effect size power analysis was calculated using Cohen’s dZ measure. The critical alpha level for all analyses was set < 0.05. All the analyses were performed using the Statistical Package for Social Sciences (SPSS Inc, version 25).
Results
Descriptive analyses on demographic (i.e. gender, age, years of schooling), clinical (i.e. disease duration, Levodopa Equivalent Daily Dose, UPDRS-III, H&Y) and cognitive (MoCA) features at T0 are reported in Table 1. The percentage of PD patients which contracted the COVID-19 virus at T1 in was 9.5% (4 out of 42).
Results of the paired Wilcoxon signed-rank test (Table 2) showed significant main effects of time of the assessment (T0 vs. T1) on the emotional subscale of the DAS, Z = − 2.49; p = 0.013; Cohen dz = 0.691, with higher scores at T0 compared to T1. A main effect of time of the assessment was also found on LEDD, Z = − 2.63; p = 0.008; Cohen dz = 0.731, and TEPS total score, Z = − 2.38; p = 0.025; Cohen dz = 0.621, with higher scores at T1 compared to T0. Results of the paired Wilcoxon signed-rank test did not show any other main effect on clinical and behavioural features (all p > 0.05).
Discussion
The main aim of the present study was to explore the impact of COVID-19 pandemic on the most frequent non-motor symptoms, such as depression, apathy, anxiety and anhedonia, in patients with PD by using symptom-specific scales. Overall, results showed a reduction of emotional apathy and anhedonia compared to the pre-COVID condition. These results seemed surprising since some recent studies suggested that PD patients may be more susceptible for negative psychological and psychosocial effects of the isolation and other restrictions due to pandemic [4]. A similar study [14] showed an impairment of non-motor symptoms in PD patients during the lockdown, in particular anxiety and cognition.
On the other hand, a study by HØrmann Thomsen et al. [15] found that PD patients experienced less sleep disturbances during the COVID-19 compared to pre-COVID-19 period. In addition, the authors revealed an improvement in HRQoL, despite increased anxiety. In the abovementioned study, participants indicated that the improvement in quality of life was due to not having usual social pressures, enjoying life being simple and slow and enjoying being at home.
A recent review [16] highlighted that also other studies found no significant worsening of mental health problems or only a minority of participants reporting worsening mental health symptoms.
Our results which, on the surface, might been positive should be explained by the fact that PD causes many people to withdraw from their social roles causing deficits in corresponding activities. Indeed, PD symptoms (e.g., motor symptoms, neuropsychiatric symptoms) negatively interfered with relationships [17]. Therefore, many PD patients already live in a state of social isolation and, paradoxically, social restrictions due to pandemic could have a less impact on this patient population. Moreover, for the purpose of our work, it is necessary to highlight that all patients included in the study were discharged from rehabilitative interventions before COVID-19 and they all lived at home with a caregiver.
Considering these issues, it is possible to hypothesize that PD patients experienced lower social pressure and benefited from greater social support from their caregiver and, as a consequence, they also experienced a reduction of emotional apathy and anhedonia.
As for clinical aspects, we observed an increase of LEDD from T0 to T1; this increase might be associated with the improvement of emotional apathy and anhedonia although we did not observed any association between the increase of LEDD and levels of apathy and anhedonia (data not shown) Moreover, nowadays, the efficacy of dopaminergic treatment on reduction of non-motor symptoms in PD is controversial and this issue deserves to be better investigated [18].
Before concluding, it should be noted that one possible limitation of our study is that our sample size was relatively small and not entirely representative of the PD population. Thus, our results do not allow for an advance in any conclusion regarding the impact of COVID-19 on PD in general. However, the findings of the present study point to the urgency of taking into account that in chronic illness, including in PD, symptoms impact the ability to fulfil social roles, causing social isolation and loneliness which are risk factors for increased health care costs and mortality [17]. The abovementioned evidence highlights the need of a consistent and persistent social support which might be represented by caregivers or/and social assistive robotics.
Change history
22 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Brooks SK, Webster RK, Smith LE et al (2020) The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 395(10227):912–920. https://doi.org/10.1016/S0140-6736(20)30460-8
Schirinzi T, Cerroni R, Di Lazzaro G et al (2020) Self-reported needs of patients with Parkinson’s disease during COVID-19 emergency in Italy. Neurol Sci 41(6):1373–1375. https://doi.org/10.1007/s10072-020-04442-1
Shalash A, Roushdy T, Essam M et al (2020) Mental health, physical activity, and quality of life in Parkinson’s disease during COVID-19 pandemic. Mov Disord 35(7):1097–1099. https://doi.org/10.1002/mds.28134
Helmich RC, Bloem BR (2020) The impact of the COVID-19 pandemic on Parkinson’s disease: hidden sorrows and emerging opportunities. J Parkinsons Dis 10(2):351–354. https://doi.org/10.3233/JPD-202038
Torrisi SA, Leggio GM, Drago F, Salomone S (2019) Therapeutic challenges of post-traumatic stress disorder: focus on the dopaminergic system. Front Pharmacol 10:404. https://doi.org/10.3389/fphar.2019.00404
De Micco R, Siciliano M, Sant’Elia V et al (2020) Correlates of psychological distress in patients with Parkinson’s disease during the COVID-19 outbreak. Mov Disord Clin Pract 8(1):60–68. https://doi.org/10.1002/mdc3.13108
D’Iorio A, Vitale C, Piscopo F et al (2017) Impact of anxiety, apathy and reduced functional autonomy on perceived quality of life in Parkinson’s disease. Parkinsonism Relat Disord 43:114–117. https://doi.org/10.1016/j.parkreldis.2017.08.003
Santangelo G, Siciliano M, Pedone R et al (2015) Normative data for the Montreal cognitive assessment in an Italian population sample. Neurol Sci 36(4):585–591. https://doi.org/10.1007/s10072-014-1995-y
Santangelo G, Falco F, D’Iorio A et al (2016) Anxiety in early Parkinson’s disease: validation of the Italian observer-rated version of the Parkinson Anxiety Scale (OR-PAS). J Neurol Sci 367:158–161. https://doi.org/10.1016/j.jns.2016.06.008
Santangelo G, D’Iorio A, Piscopo F et al (2017) Assessment of apathy minimising the effect of motor dysfunctions in Parkinson’s disease: a validation study of the dimensional apathy scale. Qual Life Res 26(9):2533–2540. https://doi.org/10.1007/s11136-017-1569-6
Stratta P, Pacifico R, Riccardi I, Daneluzzo E, Rossi A (2011) Il piacere anticipatorio e consumatorio: Uno studio di validazione della versione italiana della temporal experience of pleasure scale. Ital J Psychopathol 17:322–327
Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N (1997) The PDQ-8: development and validation of a short- form Parkinson’s disease questionnaire. Psychol Health 12:805–814. https://doi.org/10.1080/08870449708406741
Palermo G, Tommasini L, Baldacci F, Del Prete E, Siciliano G, Ceravolo R (2020) Impact of coronavirus disease 2019 pandemic on cognition in Parkinson’s disease. Mov Disord 35(10):1717–1718. https://doi.org/10.1002/mds.28254
HØrmann Thomsen T, Wallerstedt SM, Winge K, Bergquist F (2021) Life with Parkinson’s disease during the COVID-19 pandemic: the pressure is “OFF.” J Parkinsons Dis 11(2):491–495. https://doi.org/10.3233/JPD-202342
Brooks SK, Weston D, Greenberg N (2021) Social and psychological impact of the COVID-19 pandemic on people with Parkinson’s disease: a scoping review. Public Health 199:77–86. https://doi.org/10.1016/j.puhe.2021.08.014
Perepezko K, Hinkle JT, Shepard MD et al (2019) Social role functioning in Parkinson’s disease: a mixed-methods systematic review. Int J Geriatr Psychiatry 34(8):1128–1138. https://doi.org/10.1002/gps.5137
Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D (2015) Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci 10(2):227–237. https://doi.org/10.1177/1745691614568352
Lee TK, Yankee EL (2021) A review on Parkinson’s disease treatment. Neuroimmunol Neuroinflamm 8:222–244
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All participants gave their written informed consent to participate to the study, which was approved by the local ethics committee, performed in accordance to the ethical standards laid down in the 1964 Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
D’Iorio, A., Baiano, C., Maraucci, G. et al. A longitudinal study on the effects of COVID-19 pandemic on non-motor symptoms in Parkinson’s disease. Neurol Sci 43, 4605–4609 (2022). https://doi.org/10.1007/s10072-022-06112-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06112-w