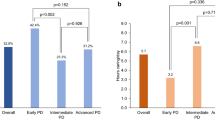
Abstract
Background
Parkinson’s disease (PD) is typically considered as a disease of the elderly. However, there is a sizeable subgroup of patients where PD starts at a younger age, known as young-onset PD (YOPD). We evaluated the differences in quality of life and caregiver strain between YOPD and later onset PD (LOPD) patients in a large cohort.
Methods
In collaboration with the Parkinson Foundation Quality Improvement Initiative (PF-QII), we conducted a retrospective three-year analysis on 962 PD patients of the QII database (starting date May 2016). Of these, 272 patients had YOPD, and 690 had LOPD. The Parkinson’s Disease Questionnaire-39 (PDQ-39) total score served as primary outcome measure. Furthermore, we analysed group differences in modified caregiver strain index (MCSI) total score, three cognition functions, and number of falls. A regression analysis adjusting for covariates was used to assess the association of age at onset with PDQ-39 and MCSI.
Results
PDQ scores were better in YOPD patients, MCSI scores on social constraint were lower in YOPD patients, but scores on financial constraint were higher in this group. After adjusting for covariates, YOPD patients had better quality of life and less caregiver strain at all follow-up moments, but not at baseline. Decline over time for all outcomes was lower in the YOPD group compared to the LOPD group. Cognitive functioning and number of falls progressed slower in the YOPD group compared to the LOPD group.
Conclusion
Compared to LOPD patients, YOPD patients had a better quality of life, less caregiver strain, fewer falls and better cognitive functioning after their first follow-up visit, and also a slower decline over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is associated with a great impact on quality of life of patients [1, 2]. Quality of life of PD patients is mainly dependent on disease severity and disability, as well as neuropsychiatric symptoms like depression [1,2,3,4]. Besides these symptoms, the gradual loss of autonomy leads to many alterations in the lives of patients and their spouses [5]. PD produces a progressive strain on spouses of patients when they become informal caregivers during disease progression [6, 7]. Earlier cross-sectional studies showed young-onset PD (YOPD) patients having poorer emotional well-being and lower quality of life [8, 9]. However, we hypothesize that YOPD patients, after being initially more struck by their diagnosis, have better capabilities to adapt to their disease and a stronger social network, resulting in better quality of life over time, as studies into other diseases with a marked impact on quality of life have pointed out [10,11,12]. We will specifically study the relation between quality of life, caregiver strain and YOPD, which thus far remains unclear. Insights into quality of life in relation with disease progression could improve care delivery and optimise the support for this often overlooked group of patients.
Subjects and methods
We performed a cross-sectional and longitudinal retrospective analysis on 8334 PD patients of the Parkinson’s Foundation Quality Improvement Initiative (PF QII) database as of May 2016. All PD patients from the participating centers were eligible for inclusion. From this cohort, we selected all patients with an age at onset under 50 (YOPD) and those with age above 70, we called late-onset PD (LOPD). The PF QII study is one of the first large-scale studies describing quality of care amongst Parkinson’s disease patients seen in 24 international PF Centres of Excellence. Registry was done by administering a questionnaire by a qualified nurse, research coordinator or doctor, consisting of multiple categories including, amongst others, Parkinson’s Disease Questionnaire-39 (PDQ-39), Modified Caregiver Strain Index (MCSI), cognitive functioning, Timed Up and Go Test (TUGT), number of falls, type and number of medication [13]. All patients and caregivers signed informed consent before being admitted.
Measures
Patients included in the PF QII-study completed a form, consisting of a section ‘patient data’, ‘patient diagnosis and PD stage’, ‘comorbid conditions’, ‘medication’, ‘other therapies’ and ‘clinical condition/outcomes’. A copy of the form can be found in the appendix. The primary outcome is PDQ-39 total score. Secondary outcomes are MCSI total score, cognitive functioning, and number of falls. The PDQ-39 is a 39-item validated scale completed by PD patients [14]. It contains eight dimensions, mobility, daily activities, emotional well-being, stigma, social support, cognition, communication and bodily discomfort. Out of each score for these dimensions one total score is calculated. It ranges from 0 to 100 where higher scores correlate with poorer Quality of Life. The MCSI is a questionnaire containing six dimensions of constraint, e.g., physical, social, financial, time, interpersonal strain and other demanding/manipulative behaviour. Scores range from 0 to 4 depending on the level of strain on caregivers. The scores for each domain are added up for a total score up to 72 points. Only this total score is used for analysis. Higher scores indicate more strain. The outcome cognition is based on standardized cognition tests, e.g., three cognition functions, immediate five-word recall, verbal fluency and delayed five-word recall. The number of falls is based on patient-derived information and categorized in ‘none/rarely’ and ‘at least monthly’.
Data analysis
We performed a cross-sectional and longitudinal analysis on 8334 PD patients of the QII database as of May 2016. We excluded patients with missing age at onset, patients aged between 50 and 70 years old (n = 5316), with neurological comorbidities (n = 158) and patients with a disease duration longer than 5 years after onset (n = 1481). We included 272 patients with YOPD, and 690 patients with LOPD. For the longitudinal analysis, we included records at baseline, 1-, 2- and 3-years after follow-up. Total sample sizes at each year after baseline were n = 962, n = 437, n = 344 and n = 244 (Fig. 1).
Univariable data analysis was done using Student’s t tests. Multiple regression analysis was used for the cross-sectional as well as for the longitudinal data to adjust for disease duration, number of symptomatic co-morbidities, and number of medications used before baseline visit. A mixed model with subjects as random effects and unstructured covariance was used to calculate fixed effect estimates. The effect of each dependent variable on the outcome scores was calculated to show the relation between each other. Results for cognitive tests were presented as standardized z-scores.
Results
Descriptive statistics
YOPD patients had a higher weight (81.9 + − 19.7 kg vs. 74.7 + − 14.4 kg, p < 0.0001) without significant difference in BMI. They were more often men (67.6% vs. 60.7%, p = 0.046) and living at home (99.6% vs. 93.8%, p < 0.001). Regular care partners were more often spouses or relatives (80.5% vs. 67.2%, p < 0.001) and less often paid caregivers or other relatives (5.2% vs. 12.3%, p < 0.001).
LOPD patients had a slightly earlier objective (physician-based) assessment after onset of first subjective symptoms than YOPD patients (2.9 + − 1.4 vs. 3.4 + − 1.3 years, p < 0.0001). The Hoehn and Yahr (HY) stage of the LOPD group was higher compared to the YOPD group. There were significantly more symptomatic co-morbidities (excluding neurological co-morbidities) in the LOPD group (0.6 + − 0.6 vs. 0.2 + − 0.5, p < 0.0001).
There was no significant difference in falls. YOPD patients had a better TUG (10.2 + − 5.6 vs. 16.2 + − 8.9, p < 0.0001) and had higher cognitive function scores (p < 0.0001). The total PDQ-39 score was not significantly different between groups (19.9 + − 15.2 vs. 21.0 + − 14.3, p = 0.299), as was the total MCSI score (13.9 + − 13.8 vs. 16.0 + − 13.9, p = 0.137) (Table 1). However, looking at PDQ39 and MCSI sub-scores, YOPD patients had a significantly better mobility, ADL, emotional status, social support and cognition scores. Social constraints were higher, but financial constraints were lower in this group.
YOPD patients were less often than LOPD patients treated with any form of Levodopa, and there was higher use of dopamine agonists, MAO-B inhibitors and amantadine, but less use of cognitive enhancers (p < 0.001). YOPD patients were less likely to be on other treatments before the first visit, with significant differences in physical, occupational and speech therapy, and with more patients having deep brain stimulation (supplementary table 1.).
Cross-sectional results
Linear regression analyses unadjusted for covariates showed no significant differences on baseline for PDQ39, MCSI and falls. YOPD patients did perform better on cognitive functioning for all three tests (p < 0.001). After adjustment for disease duration, number of symptomatic co-morbidities and number of medications, there was an additional significant difference for probability of falls in favour of YOPD patients (OR 0.51, p = 0.029; Table 2).
Longitudinal results
Analyses unadjusted for covariates showed no group differences for PDQ-39, MCSI and falls, except for MCSI score after 3 years of follow-up. Differences for standardized cognitive tests showed significant differences at follow-up after 1, 2 and 3 years after baseline (p < 0.0001; Table 3).
After adjusting for covariates, PDQ-39 scores were significantly lower for YOPD patients at all three follow-up moments. PDQ-39 score differences between groups worsened from − 2.26 (p = 0.025) after 1 year of follow-up to − 5.37 (p < 0.001) after 3 years of follow-up. The same can be seen for the results for MCSI scores (Table 2). Differences in MCSI score increased from − 3.579 (p = 0.011) after 1 year of follow-up to − 7.161 (p = 0.002) after 3 years of follow-up.
The number of falls increased in both groups over time, with the YOPD group having a lower risk of falls during follow-up (OR 0.51–0.18, p < 0.05). YOPD patients performed better for all three cognitive tests over time, with increasing mean differences for standardized cognition scores (Fig. 2).
Discussion
YOPD patients had better PDQ-39, better MCSI scores, fewer falls and better cognitive functioning after the first visit, with increasing differences between groups over time, supporting our hypothesis of YOPD patients having better quality of life and less caregiver strain.
Our findings extend similar experiences with other diseases (breast cancer and endometriosis) that also have a high impact on quality of life [10, 11, 15]. Earlier studies into age-related differences for PDQ-39 scores in PD patients showed a negative impact of earlier onset on quality of life [8, 9]. It is postulated that this is due to a variety of factors including marital conflicts, difficulties with family live, social isolation and loss of occupation [16]. However, these studies used smaller groups for young-onset PD patients with partially self-referred patients, as well adjusting for different confounders and were often cross-sectional. When compared to the general population, there is evidence that YOPD patients have greater worsening of quality of life mostly due to physical limitations and role expectation [17].
Comorbidities and medication use are important influencing factors of quality of life [18, 19]. In line with other studies, LOPD patients had more comorbidities compared to YOPD patients, possibly leading to confounding. Most importantly, we have performed multivariate analyses, adjusting for comorbidities and medication use. The difference between symptomatic and asymptomatic comorbidities can be subtle. To reduce bias, all comorbidities were assessed by the researcher, and discussed with the treating physician if needed.
Caregiver strain is not well documented in relation to YOPD. Increased caregiver burden is associated with patients’ advancing age [20, 21]. Our results support this relation between age of onset and caregiver strain, with older patients having a higher burden compared to YOPD patients. Also, earlier studies suggested that quality of life of patients and caregiver strain are strongly correlated to each other, MCSI and PDQ39 scores showing similar trends over time. Although the literature has little good-quality studies, we can conclude that caregiver strain is increased in people confronted with PD. There is some evidence for interventions to reduce caregiver strain which include education for the person with PD and the caregiver, psychotherapy targeting psychiatric symptoms in the caregiver and management of neuropsychiatric symptoms in the person living with PD [22]. Lastly, caregiver strain is influenced by cultural perceptions of disease and caregiving, leading to different results in other parts of the world [23, 24].
Our study suggests that YOPD patients experience fewer falls from baseline to the end of follow-up, similar to other large studies into predictors of falls in PD [12, 25]. A recent study using data from the PF QII-study reported no effect of age on falls. However, since we did not adjust for disease variables like HY-stage, our outcomes could be explained by differences in disease characteristics of the YOPD group, more closely resembling the general YOPD population. Fall diaries, electronically or paper based are the golden standard to measure falls [26], but impose a significant burden for patients. This study refrained from use of these instruments, possibly leading to underestimation of the number of falls. Nevertheless, the dichotomous outcome categorizing falls in ‘none/rarely’ and ‘at least monthly’, likely decreasing recall bias and subsequent underestimation of the number of falls.
The Parkinson’s Foundation QII-study is the largest international prospective study into quality of life of patients with PD. Earlier studies have shown a significant impact of the diagnosis of PD on patients and their caregivers with a difference between YOPD and LOPD, but only cross-sectionally [27]. This is the first study demonstrating longitudinal differences in quality of life, caregiver strain and falls. There are limitations of this study. There is a significant loss to follow-up for the longitudinal analysis, leading to possible attrition bias. We have analysed data cross-sectionally as well as longitudinally, for a relatively short duration of 3 years. The MCSI has not been validated for measuring caregiver strain among PD patients. However, many other studies demonstrated its reliability and validity for use in an elderly population [28]. The QII questionnaire does not report on genetic substrates of PD, or more extensively on motor symptoms (with for example the UPDRS-m) or the effects of caregiver strain. Nonetheless, we did report multiple on multiple subgroups of the latter, including physical strain (supplementary table 1.). Lastly, we did not report on employment status for the same reason. We feel our results support more extensive research into this topic.
In conclusion, this study described that YOPD patients are a group with a significantly better quality of life and slower disease progression over time compared to LOPD patients. This offers some consolation for the future perspectives for YOPD patients and creates the possibility of bringing a positive message for these patients in daily clinical practice.
References
Soh SE, Morris ME, McGinley JL (2011) Determinants of health-related quality of life in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord 17(1):1–9
Uitti RJ (2012) Treatment of Parkinson’s disease: focus on quality of life issues. Parkinsonism Relat Disord 18(Suppl 1):S34–S36
Leonardi M, Raggi A, Pagani M, Carella F, Soliveri P, Albanese A, Romito L (2012) Relationships between disability, quality of life and prevalence of nonmotor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 18(1):35–39
Muller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB (2013) Importance of motor vs non-motor symptoms for health-related quality of life in early Parkinson’s disease. Parkinsonism Relat Disord 19(11):1027–1032
Grun D, Pieri V, Vaillant M, Diederich NJ (2016) Contributory factors to caregiver burden in Parkinson disease. J Am Med Direct Assoc 17(7):626–632
Oguh O, Kwasny M, Carter J, Stell B, Simuni T (2013) Caregiver strain in Parkinson’s disease: national Parkinson Foundation Quality Initiative study. Parkinsonism Relat Disord 19(11):975–979
Lokk J (2008) Caregiver strain in Parkinson’s disease and the impact of disease duration. Eur J Phys Rehabilit Med 44(1):39–45
Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M (2003) Young- versus older-onset Parkinson’s disease: impact of disease and psychosocial consequences. Mov Disord 18(11):1250–1256
Knipe MD, Wickremaratchi MM, Wyatt-Haines E, Morris HR, Ben-Shlomo Y (2011) Quality of life in young- compared with late-onset Parkinson’s disease. Disord Off J Mov Disord Soc 26(11):2011–2018
Lovkvist L, Bostrom P, Edlund M, Olovsson M (2016) Age-related differences in quality of life in Swedish Women with Endometriosis. J Women’s Health 25(6):646–653
Park BW, Lee S, Lee AR, Lee KH, Hwang SY (2011) Quality of life differences between younger and older breast cancer patients. J Breast Cancer 14(2):112–118
Chou KL, Elm JJ, Wielinski CL, Simon DK, Aminoff MJ, Christine CW, Liang GS, Hauser RA, Sudarsky L, Umeh CC, Voss T, Juncos J, Fang JY, Boyd JT, Bodis-Wollner I, Mari Z, Morgan JC, Wills AM, Lee SL, Parashos SA (2017) Factors associated with falling in early, treated Parkinson’s disease: the NET-PD LS1 cohort. J Neurol Sci 377:137–143
Okun MS, Siderowf A, Nutt JG, O’Conner GT, Bloem BR, Olmstead EM, Guttman M, Simuni T, Cheng E, Cohen EV, Parashos S, Marsh L, Malaty IA, Giladi N, Schmidt P, Oberdorf J (2010) Piloting the NPF data-driven quality improvement initiative. Parkinsonism Relat Disord 16(8):517–521
Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N (1997) The Parkinson’s disease questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 26(5):353–357
Sturm JW, Donnan GA, Dewey HM, Macdonell RAL, Gilligan AK, Srikanth V, Thrift AG (2004) Quality of life after stroke. North East Melb Stroke Incidence NEMESIS 35(10):2340–2345
Mehanna R, Jankovic J (2019) Young-onset Parkinson’s disease: Its unique features and their impact on quality of life. Parkinsonism Relat Disord 65:39–48
Schrag A, Jahanshahi M, Quinn N (2000) How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord 15(6):1112–1118
Martinez-Martin P, Deuschl G (2007) Effect of medical and surgical interventions on health-related quality of life in Parkinson’s disease. Mov Disord 22(6):757–765
Fereshtehnejad SM, Shafieesabet M, Farhadi F, Hadizadeh H, Rahmani A, Naderi N, Khaefpanah D, Shahidi GA, Delbari A, Lökk J (2015) Heterogeneous determinants of quality of life in different phenotypes of Parkinson’s disease. PLoS ONE 10(9):e0137081
Razali R, Ahmad F, Rahman FN, Midin M, Sidi H (2011) Burden of care among caregivers of patients with Parkinson disease: a cross-sectional study. Clini Neurol Neurosurg 113(8):639–643
Henry RS, Lageman SK, Perrin PB (2020) The relationship between Parkinson’s disease symptoms and caregiver quality of life. Rehabil Psychol 65(2):137–144
Mosley PE, Moodie R, Dissanayaka N (2017) Caregiver burden in parkinson disease: a critical review of recent literature. J Geriatr Psychiatry Neurol 30(5):235–252
Smith ER, Perrin PB, Tyler CM, Lageman SK, Villaseñor T (2020) Cross-cultural differences in Parkinson’s disease caregiving and burden between the United States and Mexico. Brain Behav 1:e01753
Tanji H, Koyama S, Wada M, Kawanami T, Kurita K, Tamiya G, Saito N, Suzuki K, Kato T, Anderson KE, Gruber-Baldini AL, Fishman PS, Reich SG, Weiner WJ, Shulman LM (2013) Comparison of caregiver strain in Parkinson’s disease between Yamagata, Japan, and Maryland, The United States. Relat Disord 19(6):628–633
Hiorth YH, Larsen JP, Lode K, Pedersen KF (2014) Natural history of falls in a population-based cohort of patients with Parkinson’s disease: an 8-year prospective study. Parkinsonism Relat Disord 20(10):1059–1064
Hunter H, Rochester L, Morris R, Lord S (2018) Longitudinal falls data in Parkinson’s disease: feasibility of fall diaries and effect of attrition. Disabil Rehabil 40(19):2236–2241
Carter JH, Lyons KS, Stewart BJ, Archbold PG, Scobee R (2010) Does age make a difference in caregiver strain? Comparison of young versus older caregivers in early-stage Parkinson’s disease. Mov Disord Off J Disord Soc 25(6):724–730
Thornton M, Travis SS (2003) Analysis of the reliability of the modified caregiver strain index, the journals of gerontology Series B. Psychol Sci Soc Sci 58(2):S127–S132
Acknowledgements
We would like to thank the Parkinson’s Foundation for supporting this research, and Samuel Wu, PhD at the University of Florida. Affiliation: The Parkinson’s Foundation Quality Improvement Initiative investigators, National Parkinson Foundation, Miami, Florida, USA.
Funding
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
Under Dutch law, no additional ethical approval is required for retrospective database studies. All patients included in the QII database signed informed consent for participation in this registry.
Additional information
Institutional author: Affiliation of the The Parkinson’s Foundation Quality Improvement Initiative investigators is mentioned in Acknowlegedment section.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Te Groen, M., Bloem, B.R., Wu, S.S. et al. Better quality of life and less caregiver strain in young-onset Parkinson’s disease: a multicentre retrospective cohort study. J Neurol 268, 1102–1109 (2021). https://doi.org/10.1007/s00415-020-10266-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10266-y