Abstract
Introduction
In Parkinson’s disease (PD), non-motor fluctuations (NMFs), especially neuropsychiatric fluctuations, often coexist with motor fluctuations (MFs) but are often under-recognized by physicians and patients.
Objective
To investigate the relationship between MFs and neuropsychiatric fluctuations in PD.
Methods
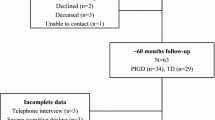
PD patients with MFs and NMFs were enrolled. The Parkinson’s Kinetigraph (PKG), a wearable device to detect MFs and dyskinesia, was used to confirm and measure MFs. The Neuropsychiatric Fluctuation Scale (NFS), a scale composed by subscores for both the ON and OFF neuropsychiatric states, was used to identify and quantify neuropsychiatric fluctuations. Patients were asked to wear the PKG for six consecutive days to identify the ON and OFF motor periods, and then to fill the NFS during the ON and OFF motor periods for three consecutive days wearing the PKG. The PKG system provided a bradykinesia score (BKS) and a dyskinesia score (DKS). Relations between BKS, DKS, and ON and OFF NFS subscores were analyzed.
Results
In 18 PD patients, anxiety, apathy, and depression characterized the OFF condition, whereas self-confidence, competency, and interest in doing things were typically in the ON condition. There was a positive correlation between the BKS and the OFF NFS subscores (p = 0.036, r = 0.51), whereas no correlation was found between the DKS and the ON NFS subscores (p = 0.38, r = 0.22).
Conclusion
Neuropsychiatric fluctuations temporarily matched the OFF MFs only in the OFF condition. These findings are useful to better manage OFF NMSs and support the need to further investigate associations between non-motor and motor symptoms in PD patients.


Similar content being viewed by others
Data availability (data transparency)
The corresponding author has full access to all data and material and can provide availability if needed.
Code availability
SPSS 22.0
References
Marsden CD, Parkes JD (1976) “ON-OFF” Effects in patients with Parkinson’s disease on chronic levedopa therapy. Lancet 307:292–296. https://doi.org/10.1016/S0140-6736(76)91416-1
Jankovic J (2005) Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord 20(Suppl 11):S11–S16. https://doi.org/10.1002/mds.20458
Hauser RA, Friedlander J, Zesiewicz TA et al (2000) A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 23:75–81. https://doi.org/10.1097/00002826-200003000-00003
Erb MK, Karlin DR, Ho BK et al (2020) mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. Npj Digit Med 3:1–10. https://doi.org/10.1038/s41746-019-0214-x
Maetzler W, Domingos J, Srulijes K et al (2013) Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov Disord 28:1628–1637
Griffiths RI, Kotschet K, Arfon S et al (2012) Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J Parkinsons Dis 2:47–55. https://doi.org/10.3233/JPD-2012-11071
Witjas T, Kaphan E, Azulay JP et al (2002) Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology 59:408–413. https://doi.org/10.1212/WNL.59.3.408
Storch A, Schneider CB, Wolz M et al (2013) Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 80:800–809. https://doi.org/10.1212/WNL.0b013e318285c0ed
Seki M, Takahashi K, Uematsu D et al (2013) Clinical features and varieties of non-motor fluctuations in Parkinson’s disease: a Japanese multicenter study. Park Relat Disord 19:104–108. https://doi.org/10.1016/j.parkreldis.2012.08.004
Storch A, Schneider CB, Klingelhöfer L et al (2015) Quantitative assessment of non-motor fluctuations in Parkinson’s disease using the Non-Motor Symptoms Scale (NMSS). J Neural Transm 122:1673–1684. https://doi.org/10.1007/s00702-015-1437-x
Goetz CG, Tilley BC, Shaftman SR et al (2008) Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170. https://doi.org/10.1002/mds.22340
Martinez-Martin P, Hernandez B (2012) The Q10 questionnaire for detection of wearing-off phenomena in Parkinson’s disease. Park Relat Disord 18:382–385. https://doi.org/10.1016/j.parkreldis.2011.12.011
Schmitt E, Krack P, Castrioto A et al (2018) The Neuropsychiatric Fluctuations Scale for Parkinson’s disease: a pilot study. Mov Disord Clin Pract 5:265–272. https://doi.org/10.1002/mdc3.12607
Postuma RB, Berg D, Stern M et al (2015) UC San Diego UC San Diego previously published works title MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Nasreddine ZS, Patel BB (2016) Validation of Montreal Cognitive Assessment, MoCA, Alternate French Versions. Can J Neurol Sci 43:665–671. https://doi.org/10.1017/CJN.2016.273
Mattis S Mattis, S (1976) Mental status examination for organic mental syndrome in the elderly patient. In Bellack, L. and Karusu, T.B., Eds., Geriatric Psychiatry, Grune & Stratton, New York, 77–121. - References - Scientific Research Publishing
Schmidt R, Freidl W, Fazekas F et al (1994) The Mattis Dementia Rating Scale: normative data from 1,001 healthy volunteers. Neurology 44:964–966. https://doi.org/10.1212/wnl.44.5.964
Goetz CG, Fahn S, Martinez-Martin P et al (2007) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 22:41–47. https://doi.org/10.1002/mds.21198
Marsden C, Schachter M (1981) Assessment of extrapyramidal disorders. Br J Clin Pharmacol 11:129–151. https://doi.org/10.1111/j.1365-2125.1981.tb01118.x
Hughes AJ, Frankel JP, Kempster PA et al (1994) Motor response to levodopa in patients with parkinsonian motor fluctuations: a follow-up study over three years. J Neurol Neurosurg Psychiatry 57:430–434. https://doi.org/10.1136/jnnp.57.4.430
Braybrook M, O’Connor S, Churchward P et al (2016) An ambulatory tremor score for Parkinson’s disease. J Parkinsons Dis 6:723–731. https://doi.org/10.3233/JPD-160898
Ossig C, Sippel D, Fauser M et al (2016) Assessment of nonmotor fluctuations using a diary in advanced Parkinson’s disease. J Parkinsons Dis 6:597–607. https://doi.org/10.3233/JPD-150764
Ossig C, Sippel D, Fauser M et al (2017) Timing and kinetics of nonmotor fluctuations in advanced Parkinson’s disease. J Parkinsons Dis 7:325–330. https://doi.org/10.3233/JPD-160996
Espay AJ, Bonato P, Nahab FB et al (2016) Technology in Parkinson’s disease: challenges and opportunities. Mov Disord 31:1272–1282. https://doi.org/10.1002/mds.26642
Odin P, Chaudhuri KR, Volkmann J et al (2018) Viewpoint and practical recommendations from a movement disorder specialist panel on objective measurement in the clinical management of Parkinson’s disease. Npj Park Dis 4:14. https://doi.org/10.1038/s41531-018-0051-7
Joshi R, Bronstein JM, Keener A et al (2019) PKG movement recording system use shows promise in routine clinical care of patients with Parkinson’s disease. Front Neurol 10:1027. https://doi.org/10.3389/fneur.2019.01027
Antonini A, Stoessl AJ, Kleinman LS et al (2018) Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin 34:2063–2073. https://doi.org/10.1080/03007995.2018.1502165
Cantello R, Gilli M, Riccio A, Bergamasco B (1986) Mood changes associated with “end-of-dose deterioration” in Parkinson’s disease: a controlled study. J Neurol Neurosurg Psychiatry 49:1182–1190. https://doi.org/10.1136/jnnp.49.10.1182
Maricle RA, Nutt JG, Valentine RJ, Carter JH (1995) Dose-response relationship of levodopa with mood and anxiety in fluctuating parkinson’s disease: a double-blind, placebo-controlled study. Neurology 45:1757–1760. https://doi.org/10.1212/WNL.45.9.1757
Maricle RA, Valentine RJ, Carter J, Nutt JG (1998) Mood response to levodopa infusion in early Parkinson’s disease. Neurology 50:1890–1892. https://doi.org/10.1212/WNL.50.6.1890
Chaudhuri KR, Yates L, Martinez-Martin P (2005) The non-motor symptom complex of Parkinson’s disease: a comprehensive assessment is essential. Curr Neurol Neurosci Rep 5:275–283
Kulisevsky J, Avila A, Barbanoj M et al (1996) Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain 119:2121–2132. https://doi.org/10.1093/brain/119.6.2121
Cáceres-Redondo MT, Carrillo F, Lama MJ et al (2014) Long-term levodopa/carbidopa intestinal gel in advanced Parkinson’s disease. J Neurol 261:561–569. https://doi.org/10.1007/s00415-013-7235-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
A written informed consent was obtained from all subjects before the inclusion in the study.
Consent for publication
All authors have approved the version to be published.
Conflict of interest
The authors declare no competing interests.
Informed consent
A written informed consent was obtained from all subjects and this information was included in the methods section.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Del Prete, E., Schmitt, E., Meoni, S. et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease?. Neurol Sci 43, 3641–3647 (2022). https://doi.org/10.1007/s10072-021-05833-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05833-8