Abstract
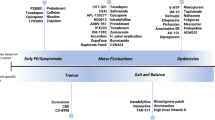
Parkinson’s disease (PD) is a neurodegenerative multisystem disorder characterized by progressive motor symptoms such as bradykinesia, tremor and muscle rigidity. Over the course of the disease, numerous non-motor symptoms, sometimes preceding the onset of motor symptoms, significantly impair patients’ quality of life. The significance of non-motor symptoms may outweigh the burden through progressive motor incapacity, especially in later stages of the disease. The advanced stage of the disease is characterized by motor complications such as fluctuations and dyskinesias induced by the long-term application of levodopa therapy. In recent years, it became evident that various non-motor symptoms such as psychiatric symptoms, fatigue and pain also show fluctuations after chronic levodopa therapy (named non-motor fluctuations or NMFs). Although NMFs have moved into the focus of interest, current national guidelines on the treatment of PD may refer to non-motor symptoms and their management, but do not mention NMF, and do not contain recommendations on their management. The present article summarizes major issues related to NMF including clinical phenomenology and pathophysiology, and outlines a number of open issues and topics for future research.
Similar content being viewed by others
References
Antonini A, Yegin A, Preda C, Bergmann L, Poewe W (2015) Global long-term study on motor and non-motor symptoms and safety of levodopa-carbidopa intestinal gel in routine care of advanced Parkinson’s disease patients; 12-month interim outcomes. Parkinsonism Relat Disord 21(3):231–235
Aquino CC, Fox SH (2015) Clinical spectrum of levodopa-induced complications. Move Disord 30(1):80–89
Ballanger B, Klinger H, Eche J, Lerond J, Vallet A-E, Le Bars D, Tremblay L, Sgambato-Faure V, Broussolle E, Thobois S (2012) Role of serotonergic 1A receptor dysfunction in depression associated with Parkinson’s disease. Move Disord 27(1):84–89
Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, Bonifati V, Brooks D, Burn DJ, Colosimo C, Fanciulli A, Ferreira J, Gasser T, Grandas F, Kanovsky P, Kostic V, Kulisevsky J, Oertel W, Poewe W, Reese J-P, Relja M, Ruzicka E, Schrag A, Seppi K, Taba P, Vidailhet M (2013) EFNS/MDS-ES/ENS corrected recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol 20(1):16–34
Bohnen NI, Kaufer DI, Hendrickson R, Constantine GM, Mathis CA, Moore RY (2007) Cortical cholinergic denervation is associated with depressive symptoms in Parkinson’s disease and parkinsonian dementia. J Neurol Neurosurg Psychiatry 78(6):641–643
Boileau I, Warsh JJ, Guttman M, Saint-Cyr JA, McCluskey T, Rusjan P, Houle S, Wilson AA, Meyer JH, Kish SJ (2008) Elevated serotonin transporter binding in depressed patients with Parkinson’s disease: a preliminary PET study with 11CDASB. Move Disord 23(12):1776–1780
Braak H, Del Tredici K, Rub U, de Vos RAI, Jansen Steur ENH, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Braak H, de Vos RAI, Bohl J, Del Tredici K (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396(1):67–72
Brooks DJ, Pavese N (2011) Imaging biomarkers in Parkinson’s disease. Prog Neurobiol 95(4):614–628
Brooks DJ, Seppi K (2009) Proposed neuroimaging criteria for the diagnosis of multiple system atrophy. Move Disord 24(7):949–964
Brun L, Lefaucheur R, Fetter D, Derrey S, Borden A, Wallon D, Bourre B, Maltete D (2014) Non-motor fluctuations in Parkinson’s disease: prevalence, characteristics and management in a large cohort of parkinsonian outpatients. Clin Neurol Neurosurg 127:93–96
Cerasa A, Salsone M, Nigro S, Chiriaco C, Donzuso G, Bosco D, Vasta R, Quattrone A (2014) Cortical volume and folding abnormalities in Parkinson’s disease patients with pathological gambling. Parkinsonism Relat Disord 20(11):1209–1214
Chaudhuri KR, Sauerbier A, Rojo JM, Sethi K, Schapira AHV, Brown RG, Antonini A, Stocchi F, Odin P, Bhattacharya K, Tsuboi Y, Abe K, Rizos A, Rodriguez-Blazquez C, Martinez-Martin P (2015) The burden of non-motor symptoms in Parkinson’s disease using a self-completed non-motor questionnaire: a simple grading system. Parkinsonism Relat Disord 21(3):287–291
Chen F-X, Kang D-Z, Chen F-Y, Liu Y, Wu G, Li X, Yu L-H, Lin Y-X, Lin Z-Y (2016) Gray matter atrophy associated with mild cognitive impairment in Parkinson’s disease. Neurosci Lett 617:160–165
Cheon SM, Park MJ, Kim WJ, Kim JW (2009) Non-motor off symptoms in Parkinson’s disease. J Korean Med Sci 24(2):311–314
Dafsari HS, Reddy P, Herchenbach C, Wawro S, Petry-Schmelzer JN, Visser-Vandewalle V, Rizos A, Silverdale M, Ashkan K, Samuel M, Evans J, Huber CA, Fink GR, Antonini A, Chaudhuri KR, Martinez-Martin P, Timmermann L (2016) Beneficial effects of bilateral subthalamic stimulation on non-motor symptoms in Parkinson’s disease. Brain Stimul 9(1):78–85
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28 K) immunohistochemistry. Brain J Neurol 122(Pt 8):1421–1436
de la Fuente-Fernández R (2013) Imaging of dopamine in PD and implications for motor and neuropsychiatric manifestations of PD. Front Neurol 4:90. doi:10.3389/fneur.2013.00090
Erro R, Pappata S, Amboni M, Vicidomini C, Longo K, Santangelo G, Picillo M, Vitale C, Moccia M, Giordano F, Brunetti A, Pellecchia MT, Salvatore M, Barone P (2012) Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson’s disease patients. Parkinsonism Relat Disord 18(9):1034–1038
Fauser M, Lohle M, Ebersbach G, Odin P, Fuchs G, Jost WH, Chaudhuri KR, Koch R, Storch A (2015) Intraindividual variability of nonmotor fluctuations in advanced Parkinson’s disease. J Parkinson’s Dis 5(4):737–741
Fedorova TD, Seidelin LB, Knudsen K, Schacht AC, Geday J, Pavese N, Brooks DJ, Borghammer P (2017) Decreased intestinal acetylcholinesterase in early Parkinson disease: an 11C-donepezil PET study. Neurology 88(8):775–781
Gao L-L, Wu T (2016) The study of brain functional connectivity in Parkinson’s disease. Transl Neurodegener 5:18
Gjerloff T, Fedorova T, Knudsen K, Munk OL, Nahimi A, Jacobsen S, Danielsen EH, Terkelsen AJ, Hansen J, Pavese N, Brooks DJ, Borghammer P (2015) Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with 11C-donepezil PET. Brain J Neurol 138(Pt 3):653–663
Gottlich M, Munte TF, Heldmann M, Kasten M, Hagenah J, Kramer UM (2013) Altered resting state brain networks in Parkinson’s disease. PLoS One 8(10):e77336
Gunal Di, Nurichalichi K, Tuncer N, Bekiroglu N, Aktan S (2002) The clinical profile of nonmotor fluctuations in Parkinson’s disease patients. Can J Neurol Sci 29(1):61–64
Hea Braak (2006) Pathology associated with sporadic Parkinson’s disease—where does it end? J. Neural Transm Suppl:89–97
Hely MA, Morris JGL, Reid WGJ, Trafficante R (2005) Sydney multicenter study of Parkinson’s disease: non-l-dopa-responsive problems dominate at 15 years. Move Disord 20(2):190–199
Hillen ME, Sage JI (1996) Nonmotor fluctuations in patients with Parkinson’s disease. Neurology 47(5):1180–1183
Hu X, Song X, Li E, Liu J, Yuan Y, Liu W, Liu Y (2015) Altered resting-state brain activity and connectivity in depressed Parkinson’s disease. PLoS One 10(7):e0131133
Iacono D, Geraci-Erck M, Rabin ML, Adler CH, Serrano G, Beach TG, Kurlan R (2015) Parkinson disease and incidental Lewy body disease: just a question of time? Neurology 85(19):1670–1679
Kleiner-Fisman G, Martine R, Lang AE, Stern MB (2011) Development of a non-motor fluctuation assessment instrument for Parkinson disease. Parkinson’s Dis 2011:292719
Luo C, Chen Q, Song W, Chen K, Guo X, Yang J, Huang X, Gong Q, Shang H-F (2014) Resting-state fMRI study on drug-naive patients with Parkinson’s disease and with depression. J Neurol Neurosurg Psychiatry 85(6):675–683
Maricle RA, Nutt JG, Valentine RJ, Carter JH (1995) Dose-response relationship of levodopa with mood and anxiety in fluctuating Parkinson’s disease. A double-blind, placebo-controlled study. Neurology 45(9):1757–1760
Marsden CD, Parkes JD (1976) “On-off” effects in patients with parkinson’s disease on chronic levodopa therapy. Lancet 307(7954):292–296
Martinez-Fernandez R, Schmitt E, Martinez-Martin P, Krack P (2016) The hidden sister of motor fluctuations in Parkinson’s disease: a review on nonmotor fluctuations. Move Disord 31(8):1080–1094
Niccolini F, Su P, Politis M (2014) Dopamine receptor mapping with PET imaging in Parkinson’s disease. J Neurol 261(12):2251–2263
Nigro S, Riccelli R, Passamonti L, Arabia G, Morelli M, Nistico R, Novellino F, Salsone M, Barbagallo G, Quattrone A (2016) Characterizing structural neural networks in de novo Parkinson disease patients using diffusion tensor imaging. Hum Brain Mapp 37(12):4500–4510
Ossig C, Sippel D, Fauser M, Gandor F, Jost WH, Ebersbach G, Storch A (2016) Assessment of nonmotor fluctuations using a diary in advanced Parkinson’s disease. J Parkinson’s Dis 6(3):597–607
Ossig C, Sippel D, Fauser M, Gandor F, Jost WH, Ebersbach G, Storch A (2017) Timing and Kinetics of Nonmotor Fluctuations in Advanced Parkinson’s Disease. J Parkinson’s Dis
Pagano G, Molloy S, Bain PG, Rabiner EA, Chaudhuri KR, Brooks DJ, Pavese N (2016) Sleep problems and hypothalamic dopamine D3 receptor availability in Parkinson disease. Neurology 87(23):2451–2456
Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ (2010) Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain J Neurol 133(11):3434–3443
Picillo M, Palladino R, Moccia M, Erro R, Amboni M, Vitale C, Barone P, Pellecchia MT (2016) Gender and non motor fluctuations in Parkinson’s disease: a prospective study. Parkinsonism Relat Disord 27:89–92
Poewe W (2008) Non-motor symptoms in Parkinson’s disease. Eur J Neurol 15(Suppl 1):14–20
Politis M, Piccini P, Pavese N, Koh S-B, Brooks DJ (2008) Evidence of dopamine dysfunction in the hypothalamus of patients with Parkinson’s disease: an in vivo 11C-raclopride PET study. Exp Neurol 214(1):112–116
Politis M, Wu K, Loane C, Turkheimer FE, Molloy S, Brooks DJ, Piccini P (2010) Depressive symptoms in PD correlate with higher 5-HTT binding in raphe and limbic structures. Neurology 75(21):1920–1927
Raudino F (2001) Non motor off in Parkinson’s disease. Acta Neurol Scand 104(5):312–315
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain J Neurol 128:1314–1322
Riley DE, Lang AE (1993) The spectrum of levodopa-related fluctuations in Parkinson’s disease. Neurology 43(8):1459–1464
Rizos A, Martinez-Martin P, Odin P, Antonini A, Kessel B, Kozul TK, Todorova A, Douiri A, Martin A, Stocchi F, Dietrichs E, Chaudhuri KR (2014) Characterizing motor and non-motor aspects of early-morning off periods in Parkinson’s disease: an international multicenter study. Parkinsonism Relat Disord 20(11):1231–1235
Robertson AD, Messner MA, Shirzadi Z, Kleiner-Fisman G, Lee J, Hopyan J, Lang AE, Black SE, MacIntosh BJ, Masellis M (2016) Orthostatic hypotension, cerebral hypoperfusion, and visuospatial deficits in Lewy body disorders. Parkinsonism Relat Disord 22:80–86
Rosa-Grilo M, Qamar MA, Evans A, Chaudhuri KR (2016) The efficacy of apomorphine—a non-motor perspective. Parkinsonism Relat Disord 33(Suppl 1):S28–S35
Salsone M, Cerasa A, Arabia G, Morelli M, Gambardella A, Mumoli L, Nistico R, Vescio B, Quattrone A (2014) Reduced thalamic volume in Parkinson disease with REM sleep behavior disorder: volumetric study. Parkinsonism Relat Disord 20(9):1004–1008
Seki M, Takahashi K, Uematsu D, Mihara B, Morita Y, Isozumi K, Ohta K, Muramatsu K, Shirai T, Nogawa S, Gotoh J, Yamaguchi K, Tomita Y, Yasutomi D, Nihei Y, Iwasawa S, Suzuki N (2013) Clinical features and varieties of non-motor fluctuations in Parkinson’s disease: a Japanese multicenter study. Parkinsonism Relat Disord 19(1):104–108
Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, Hametner EM, Poewe W, Rascol O, Goetz CG, Sampaio C (2011) The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Move Disord 26(Suppl 3):S42–S80
Stacy M, Hauser R (2007) Development of a Patient Questionnaire to facilitate recognition of motor and non-motor wearing-off in Parkinson’s disease. J Neural Transm (Vienna, Austria: 1996) 114(2):211–217
Stocchi F, Antonini A, Barone P, Tinazzi M, Zappia M, Onofrj M, Ruggieri S, Morgante L, Bonuccelli U, Lopiano L, Pramstaller P, Albanese A, Attar M, Posocco V, Colombo D, Abbruzzese G (2014) Early detection of wearing off in Parkinson disease: the DEEP study. Parkinsonism Relat Disord 20(2):204–211
Stoessl AJ, Lehericy S, Strafella AP (2014) Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet 384(9942):532–544
Storch A, Schneider CB, Wolz M, Sturwald Y, Nebe A, Odin P, Mahler A, Fuchs G, Jost WH, Chaudhuri KR, Koch R, Reichmann H, Ebersbach G (2013) Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 80(9):800–809
Storch A, Schneider CB, Klingelhofer L, Odin P, Fuchs G, Jost WH, Martinez-Martin P, Koch R, Reichmann H, Chaudhuri KR, Ebersbach G (2015) Quantitative assessment of non-motor fluctuations in Parkinson’s disease using the Non-motor Symptoms Scale (NMSS). J Neural Transm (Vienna, Austria: 1996) 122(12):1673–1684
Surdhar I, Gee M, Bouchard T, Coupland N, Malykhin N, Camicioli R (2012) Intact limbic-prefrontal connections and reduced amygdala volumes in Parkinson’s disease with mild depressive symptoms. Parkinsonism Relat Disord 18(7):809–813
Treglia G, Cason E (2012) Meta-analysis on MIBG scintigraphy in differential diagnosis between Parkinson’s disease and neurodegenerative parkinsonism. Autor reply. Parkinsonism Relat Disord 18(805):806
Vriend C, Raijmakers P, Veltman DJ, van Dijk KD, van der Werf YD, Foncke EMJ, Smit JH, Berendse HW, van den Heuvel OA (2014) Depressive symptoms in Parkinson’s disease are related to reduced 123IFP-CIT binding in the caudate nucleus. J Neurol Neurosurg Psychiatry 85(2):159–164
Weintraub D, Newberg AB, Cary MS, Siderowf AD, Moberg PJ, Kleiner-Fisman G, Duda JE, Stern MB, Mozley D, Katz IR (2005) Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson’s disease. J Nucl Med 46(2):227–232
Wen M-C, Ng SYE, Heng HSE, Chao YX, Chan LL, Tan EK, Tan LCS (2016) Neural substrates of excessive daytime sleepiness in early drug naive Parkinson’s disease: a resting state functional MRI study. Parkinsonism Relat Disord 24:63–68
Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA (2002) Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology 59(3):408–413
Yao N, Pang S, Cheung C, Chang RS-K, Lau KK, Suckling J, Yu K, Mak HK-F, McAlonan G, Ho S-L, Chua S-E (2015) Resting activity in visual and corticostriatal pathways in Parkinson’s disease with hallucinations. Parkinsonism Relat Disord 21(2):131–137
Yoo K, Chung SJ, Kim HS, Choung O-H, Lee Y-B, Kim M-J, You S, Jeong Y (2015) Neural substrates of motor and non-motor symptoms in Parkinson’s disease: a resting FMRI study. PLoS One 10(4):e0125455
YorkWilliams S, Poston KL (2014) What light have resting state fMRI studies shed on cognition and mood in Parkinson’s disease? J Clin Move Disord 1:4
Acknowledgements
Funding was provided by AbbVie Deutschland.
Author information
Authors and Affiliations
Contributions
All authors participated in drafting the manuscript and its critical revision. All authors approved the manuscript prior to its submission.
Corresponding author
Ethics declarations
Conflict of interest
Joseph Classen has received unrestricted research grants from St. Jude Medical and Medtronic, and honoraria for presentations/advisory boards/consultations from Bayer Pharma, UCB, AbbVie, Desitin, Zambon, and royalties from Thieme Verlag and Kohlhammer Verlag outside the submitted work. Klaus Seppi reports grants from Medical University Innsbruck, Oesterreichische Nationalbank Nationalbank (Austrian Central Bank, Anniversary Fund; project no.: 14174), Austrian Science Fund (FWF: Der Wissenschaftsfonds; project no.: KLI82-B00), Michael J. Fox Foundation (project: PPMI study), personal fees from International Parkinson and the Movement Disorder Society, Teva, UCB, Lundbeck, AOP Orphan, Roche and Abbvie outside the submitted work. Alexander Storch has received unrestricted research grants from Boehringer Ingelheim, TEVA Pharma and Global Kinetics Cooperation (GKC, Melbourne, Australia), and honoraria for presentations/advisory boards/consultations from GKC, Pfizer Ltd. UK, Mundipharma, UCB, Grünenthal, AbbVie, TVA, MEDA, Desitin, Medtronic, Zambon, and royalties from Kohlhammer Verlag and Elsevier Press outside the submitted work. Jiri Koschel, Christian Oehlwein, Peter Urban, Christian Winkler, Ullrich Wüllner had nothing to disclose.
Rights and permissions
About this article
Cite this article
Classen, J., Koschel, J., Oehlwein, C. et al. Nonmotor fluctuations: phenotypes, pathophysiology, management, and open issues. J Neural Transm 124, 1029–1036 (2017). https://doi.org/10.1007/s00702-017-1757-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-017-1757-0